
2023-128 Department of Pesticide Regulation
Insufficient Staffing and Inefficient Processes Delay Pesticide Registrations
Published: July 2, 2024Report Number: 2023-128
July 2, 2024
2023-128
The Governor of California
President pro Tempore of the Senate
Speaker of the Assembly
State Capitol
Sacramento, CA 95814
Dear Governor and Legislative Leaders:
As directed by the Joint Legislative Audit Committee, my office conducted an audit of California’s Department of Pesticide Regulation (DPR) and its pesticide registration process. The following report details the audit’s findings and conclusions. Overall, we determined that the processing time for pesticide registration applications is lengthy and variable, and DPR’s insufficient staffing and inefficient processes have contributed to registration delays. Additionally, DPR is making efforts to raise fees to address its increasing costs.
DPR’s pesticide registration application processing times have been increasing: in 2023 the department took an average of more than 3.5 years to process registrations for pesticides with new active ingredients and major new uses, twice as long as it took in 2019. Although DPR asserts that the existing regulatory standards for the length of its data evaluations are outdated, it has not taken steps to substantively update them since 1989, even though it is required to review its regulations every five years. Long application processing times can delay the medical, agricultural, and other benefits that pesticide products provide, and it can reduce revenue for businesses providing those products.
One of the causes of DPR’s delays in processing registrations is its lack of adequate staffing, and DPR has recently taken steps to request additional positions. Nonetheless, it lacks a formal and ongoing process to determine its staffing needs. Additionally, DPR’s registration process relies on paper documentation and 24 disparate data systems, creating significant inefficiencies. DPR plans to begin implementing the first stage of a new, integrated data system in August 2024. However, addressing its staffing levels and implementing its new data system have contributed to its growing expenses. The DPR Fund balance has declined by more than $7 million in the last five years. DPR hopes to address these rising costs, in part, by raising its registration fees and its mill assessment.
Respectfully submitted,
GRANT PARKS
California State Auditor
Selected Abbreviations Used in This Report
| CalPEST | California Pesticide Electronic Submission Tracking |
| CDT | California Department of Technology |
| DPR | Department of Pesticide Regulation |
| DPR Fund | Department of Pesticide Regulation Fund |
| IT | information technology |
| SPR | special project report |
| U.S. EPA | U.S. Environmental Protection Agency |
| U.S. GAO | U.S. Government Accountability Office |
Summary
KEY FINDINGS AND RECOMMENDATIONS
As part of its mission to protect human health and the environment, California’s Department of Pesticide Regulation (DPR) evaluates and registers new pesticides with attention on the pesticide’s effects on public health and safety and the environment. Our review found the following:
- DPR’s pesticide registration application processing times have been increasing: in 2023 the department took an average of more than 3.5 years to process registrations for pesticides with new active ingredients and major new uses, twice as long as it took in 2019. Although DPR asserts that the existing regulatory standards for the length of its data evaluations are outdated, it has not taken steps to substantively update them since 1989, even though state law requires the department to review its regulations every five years. Long application processing times can delay medical, agricultural, residential, and other benefits that pesticide products provide and can reduce revenue for businesses providing those products.
- One of the causes of DPR’s delays in processing registrations is its lack of adequate staffing, and DPR has recently taken steps to request additional positions. Nonetheless, it lacks a formal and ongoing process to determine its staffing needs. Additionally, DPR’s registration process relies on paper documentation and 24 disparate data systems, creating significant inefficiencies. DPR plans to begin implementing the first stage of a new, integrated data system in August 2024, and it expects full implementation by March 2025.
- Increasing staffing levels and planned implementation of its new data system have contributed to DPR’s growing expenses, which have contributed to the Department of Pesticide Regulation Fund balance declining by more than $7 million in the last five years. DPR hopes to address these rising costs, in part, by raising its registration fees and its mill assessment.
To address these findings, we recommend that DPR establish valid and measurable standards for the time it should take to process registration applications, that it track and annually report its progress toward meeting those standards, and that it use its progress to inform ongoing evaluation of its staffing needs.
Agency Perspective
DPR explained that the report’s recommendations align with its current efforts and stated that it is in the process of implementing them.
Introduction
Background
The mission of DPR is to protect human health and the environment by regulating the sale and use of pesticides in California and by fostering reduced-risk pest management. State law defines pesticides as generally including any substance, or mixture of substances, intended for regulating plant growth or preventing or mitigating pests, such as insects, weeds, bacteria, and viruses. Pesticides are essential for the production of food and for the protection of the public’s health and safety. State law establishes DPR’s pesticide regulation programs to serve several purposes, including providing for the proper, safe, and efficient use of pesticides; protecting the environment from harmful pesticides; and ensuring that agricultural and pest control workers have safe working conditions where pesticides are present.
In fiscal year 2023–24, DPR had nearly 450 authorized staff positions and a budget of $132 million. DPR is generally organized into three divisions: the Pesticide Programs Division, the Administrative Services Division, and the Office of Technology Services. DPR’s Executive Office also has other offices, such as the Office of Legal Affairs, the Office of Environmental Justice, and the Office of Legislation and Policy. The Pesticide Programs Division is responsible for activities that include evaluating and registering new pesticides, conducting risk assessments, and evaluating pesticides in the environment. Although the majority of DPR’s resources support its other responsibilities, this audit focuses primarily on DPR’s pesticide registration process and related duties.
DPR’s Process for Registering Pesticides
Manufacturers, importers, or dealers of any pesticide must, in the majority of cases, obtain a certificate of registration from DPR before offering such pesticide for sale in California. Pesticides must also generally be registered with the U.S. Environmental Protection Agency (U.S. EPA) before DPR can register the product. Entities seeking registration may include chemical companies, government agencies, importers, or any person wishing to market a pesticide product in California. Throughout this report, we refer to any entity seeking registration or holding a certificate of registration as the registrant. To apply for registration, a registrant must submit an application, pay an application fee, provide copies of the product labeling, and include applicable supporting data.
The Pesticide Registration Branch (Registration Branch) is responsible for processing and tracking registration applications, coordinating scientific evaluations of pesticides, and ensuring that pesticides meet federal and state laws, among other duties. Registration Branch staff include regulatory scientists who review applications and initiate the steps of the registration process, as Figure 1 shows.
Figure 1
DPR’s Pesticide Registration Process
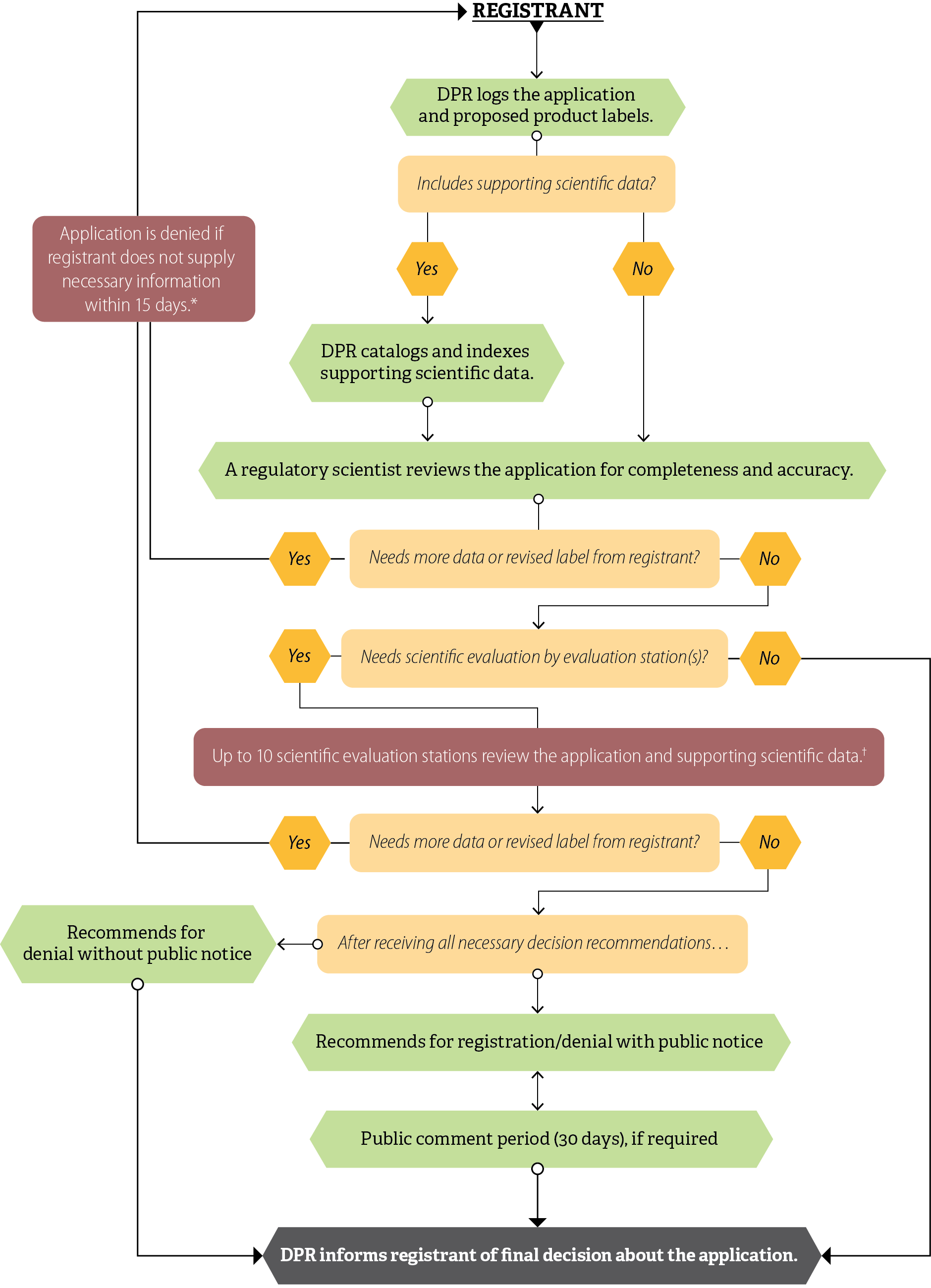
Source: DPR registration documents and state law.
* DPR established the 15-day requirement in January 2024.
† Scientific evaluations are mostly done sequentially, except for new active ingredient and major new use applications, which DPR routes simultaneously.
Figure 1 is a flowchart that represents the process an application for pesticide registration goes through from submission to DPR’s decision. It is aligned from top to bottom with boxes indicating each step or condition. At the top of the flowchart is the word “registrant”. In the first step from the registrant, the flowchart has an arrow pointing to a box that says “DPR logs the application and proposed product labels.” A line from that box connects it to a box with a condition that asks “Includes supporting scientific data?”. Lines connect that condition to two hexagons: one that read “Yes” and one that reads “No”. From the hexagon indicating “Yes” there is an arrow leading to a box with the step “DPR catalogs and indexes supporting scientific data.” Then an arrow from that step leads to a box with the step “A regulatory scientist reviews the application for completeness and accuracy.” For the previous condition asking “Includes supporting scientific data?”, the hexagon indicating “No” has an arrow leading directly to the box with the step “A regulatory scientist reviews the application for completeness and accuracy.” That step is connected by a line to a box with a condition that asks “Needs more data or revised label from registrant?” Lines connect that condition to two hexagons: one that read “Yes” and one that reads “No”. The line from the hexagon indicating “Yes” leads up the left side of the flowchart to a box that states “Application is denied if registrant does not supply necessary information within 15 days.” An asterisk is attached to that statement. At the bottom of the graphic, the asterisk states that DPR established the 15-day requirement in January 2024. The line from the hexagon continues past this box and turns right at the top to return to the word registrant at the beginning of the flow chart. For the previous condition asking “Needs more data or revised label from registrant?”, the hexagon indicating “No” has an arrow leading directly to a box with the condition “Needs scientific evaluation by evaluation station(s)?” Lines connect that condition to two hexagons: one that read “Yes” and one that reads “No.” The line from the hexagon indicating “No” leads down to the box with the final step at the end of the flow chart that says “DPR informs registrant of the final decision about the application.” For the previous condition asking “Needs scientific evaluation by evaluation station(s)?”, the line from the hexagon indicating “Yes” leads down to the box with the step “Up to 10 scientific evaluation stations review the application and supporting scientific data.” A cross symbol is attached to that statement, indicating a footnote. At the bottom of the graphic, the cross symbol footnote states that scientific evaluations are mostly done sequentially, except for new active ingredient and major new use applications, which DPR routes simultaneously. That step is connected by a line to a box with a condition that asks “Needs more data or revised label from registrant?” Lines connect that condition to two hexagons: one that read “Yes” and one that reads “No.” The line from the hexagon indicating “Yes” leads up the left side of the flowchart to the box that states “Application is denied if registrant does not supply necessary information within 15 days.” That has the line that continues past this box and turns right at the top to return to the word registrant at the beginning of the flow chart. For the previous condition asking “Needs more data or revised label from registrant?”, the hexagon indicating “No” has an arrow leading directly to a box with the step “After receiving all necessary recommendations…” Lines connect that step to two different boxes: one to the left with the step “Recommends for denial without public notice” and one below with the step “Recommends for denial/registration with public notice.” The step “recommends for denial without notice” has an arrow that connects it to the last box in the flowchart, with the step “DPR informs registrant of final decision about application.” The step “recommends for registration/denial with notice” has an arrow that points to and from the next box with the step “Public comment (30 days), if required”. That step has an arrow that connects it to a box with the last step in the flowchart, which says “DPR informs registrant of final decision about the application.”
After DPR receives the registration applications in the mail, the regulatory scientists review the application, proposed labels, and federal documentation, and determine whether the proposed product label complies with labeling requirements. If needed, evaluation scientists in other Pesticide Programs Division branches evaluate proposed labels and supporting data within as many as 10 evaluation stations, as Figure 2 shows. The number of evaluation stations that must review an application depends on the specific aspects of an application, including the type of product and the claims registrants make about it. For example, evaluation in the Chemistry station determines whether the application needs routing to the Groundwater station and evaluation in the Plant Physiology station determines whether the application needs routing to the Air station. In a selection of 20 applications we reviewed, we observed two applications that went to eight stations.
Figure 2
Scientific Evaluation Stations and Areas of Evaluation
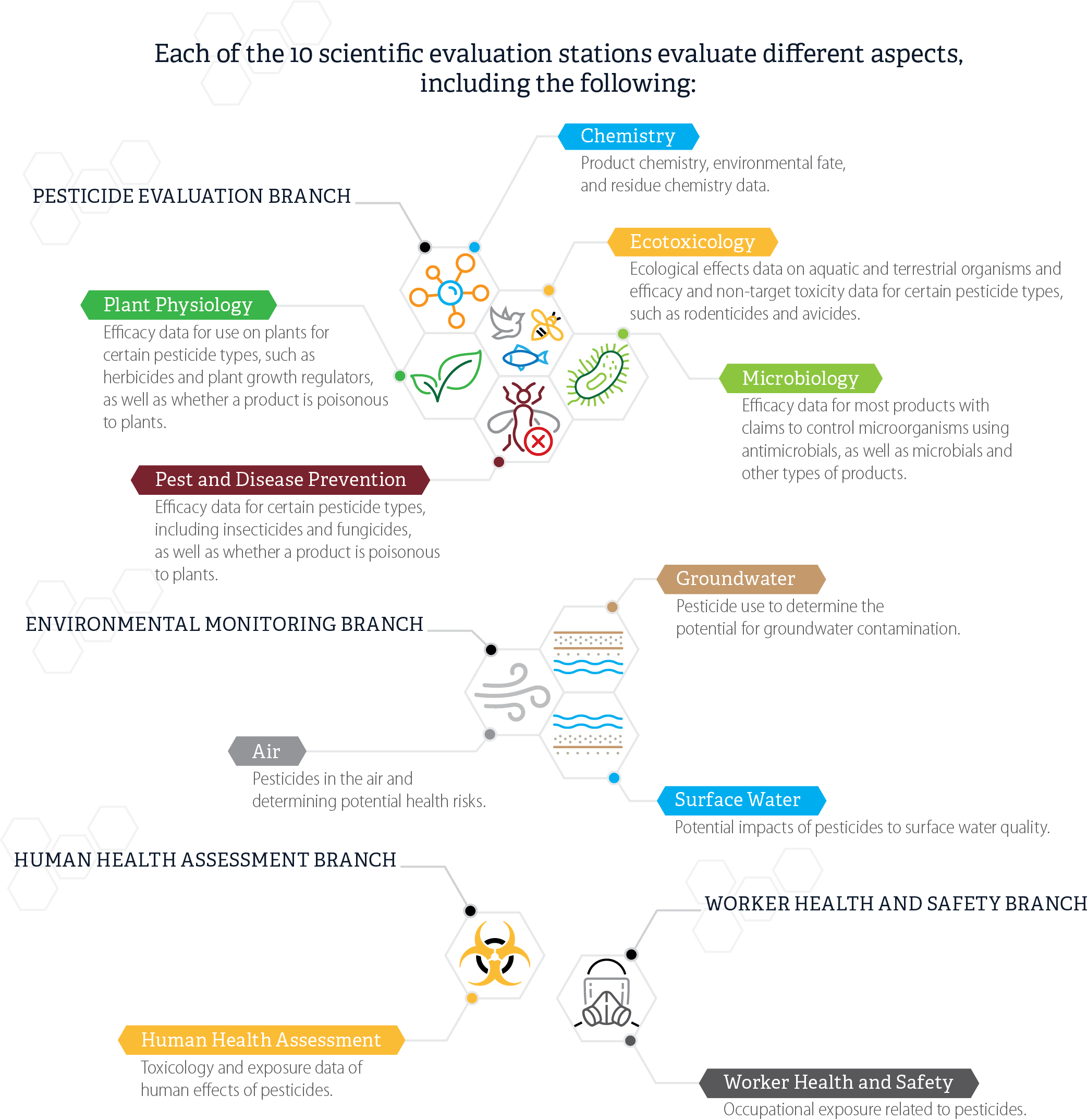
Source: Pesticide Registration Process Desk Manual.
Figure 2 displays 10 scientific evaluation stations, organized and grouped by branch, and some of the different aspects of an application each station evaluates. At the top of the figure is a heading that states that “each of the 10 scientific evaluation stations evaluate different aspects, including the following:” At the top left of the figure is the label “Pesticide Evaluation Branch”. This label is connected with a line to five connected hexagonal images. The first image is a simple generic image of a molecule. A line connects this image to a box with the station label “Chemistry”. Below that label are the words “Product chemistry, environmental fate, and residue chemistry data.” A second connected hexagonal image is a simple image of a plant. A line connects this image to a box with the station label “Plant Physiology”. Below that label are the words “Efficacy data for use on plants for certain pesticide types, such as herbicides and plant growth regulators, as well as whether a product is poisonous to plants.” A third connected hexagonal image is of a bird, a bee, and a fish. A line connects this image to a box with the station label “Ecotoxicology”. Below that label are the words “Ecological effects data on aquatic and terrestrial organisms and efficacy and non-target toxicity data for certain pesticide types, such as rodenticides and avicides.” A fourth connected hexagonal image is of a microorganism. A line connects this image to a box with the station label “Microbiology.” Below that label are the words “Efficacy data for most products with claims to control microorganisms using antimicrobials, as well as microbials and other types of products.” The fifth connected hexagonal image is of an insect with a red X mark on it. A line connects this image to a box with the station label “Pest and Disease Prevention.” Below that label are the words “Efficacy data for certain pesticide types, including insecticides and fungicides, as well as whether a product is poisonous to plants.” In the middle left of the figure is the label “Environmental Monitoring Branch.” This label is connected with a line to three connected hexagonal images. The first image is three curled lines symbolizing air. A line connects this image to a box with the station label “Air.” Below that label are the words “Pesticides in the air and determining potential health risks.” A second connected hexagonal image is of a set of brown straight lines over a set of blue curvy lines, symbolizing underground water. A line connects this image to a box with the station label “Groundwater.” Below that label are the words “Pesticide use to determine the potential for groundwater contamination.” A third connected hexagonal image is of a set of brown straight lines under a set of blue curvy lines, symbolizing above-ground water. A line connects this image to a box with the station label “Surface Water.” Below that label are the words “Potential impacts of pesticides to surface water quality.” In the bottom left of the Figure is the label “Human Health Assessment Branch.” This label is connected with a line to one hexagonal image. This hexagonal image is an image of a biohazard symbol. A line connects this image to a box with the station label “Human Health Assessment.” Below that label are the words “Toxicology and exposure data of human effects of pesticides.” In the bottom right of the figure is the label “Worker Health and Safety Branch.” This label is connected with a line to one hexagonal image. This hexagonal image is an image of a human head wearing a respirator mask. A line connects this image to a box with the station label “Worker Health and Safety.” Below that label are the words “Occupational exposure related to pesticides.”
These evaluation stations conduct an extensive scientific review of pesticide application data. This scientific evaluation must consider several factors, including whether the pesticide’s use is of less public value or greater detriment to the environment than the benefit received by its use or whether, when properly used, it is detrimental to vegetation, domestic animals, or to public health and safety. DPR’s review of scientific data must also give special attention to factors including acute health effects, such as oral or dermal toxicity; evidence of chronic health effects, such as carcinogenicity or delayed neurotoxicity; and potential for environmental damage, including interference with the attainment of applicable environmental standards.
DPR’s registration process also relies on public input. The California Environmental Quality Act generally requires DPR to produce a public report before completing registration. Each public report must contain a statement of any significant adverse environmental impact that can reasonably be expected to occur, directly or indirectly, from implementing a registration proposal, a statement of any reasonable mitigation measures that are available to minimize a significant adverse environmental impact, and a statement of reasonable alternatives that would reduce any significant environmental impact. According to DPR’s Pesticide Registration Process Desk Manual, it does not need to produce a public report if it is proposing to deny an application. If a comment about a product raises a significant human health or environmental concern, DPR’s final action on the proposed decision must include a written evaluation of the concern. DPR is prohibited from approving an activity that would cause a significant adverse environmental impact if there is a feasible alternative or feasible mitigation measures available that would substantially lessen the impact.
After the initial registration, certain changes to a product require new review. For example, a registrant may seek to change the product’s label to include additional use sites, crops, and pests or to adjust the stated percent of an active ingredient. To do so, the registrant must submit an application for amendment. Amendments may require the submission of new data or may reference substantially similar products that are already registered for use in California.
DPR Funding and Fees
DPR’s primary funding source is its Department of Pesticide Regulation Fund (DPR Fund). The DPR Fund receives revenue from three main sources: mill assessment revenue, registration fees, and licensing and certification fees. For fiscal year 2022–23, according to DPR’s accounting data, a mill assessment—an assessment imposed on the sale of registered pesticides for use in the State—accounts for 79 percent of the funding in the DPR Fund. This assessment is based on a mill, which is one-tenth of a cent. For transactions since 2004, the Legislature capped the assessment at 21 mills, or 2.1 cents, per dollar of sales of pesticides for use in California.1 The mill assessment must be paid by the registrant, unless the registrant does not know that the pesticide is or will be sold for use in the State. In such cases, the entity that first sells the pesticide for use in the State, such as a licensed pesticide broker or a licensed pest control dealer, must pay the assessment.2 State law allows DPR to use its share of the mill assessment revenue to support its operations, including the registration program when registration fees are insufficient to cover the program’s costs.
State law requires DPR to adopt regulations that set the fees for its licensing and certification program and its registration program at amounts that are sufficient to support the programs’ expenditure levels. In other words, each program should be self-supporting. The licensing and certification program provides the licensing and certification required for individuals and businesses that sell, consult on, or professionally apply pesticides. The law requires that DPR collect fees on license and certification examinations, applications, and renewals, among other related fees. Fees for the registration program include annual renewal fees, fees for new products and amendments to registered products, and late payment penalties. DPR’s accounting records for fiscal year 2022–23 show that registration fees make up about 16 percent of the DPR Fund’s revenue, and licensing fees make up 2 percent.
Issues
DPR’s Processing Time Frames for Pesticide Registration Applications Are Lengthy and Variable
DPR is Making Efforts to Raise Fees to Address Its Increasing Expenses
DPR’s Processing Time Frames for Pesticide Registration Applications Are Lengthy and Variable
Key Points
- The time it takes DPR to process registration applications has increased significantly since the COVID-19 pandemic. Its average processing time for new active ingredient or major new use applications more than doubled from 2019 to 2023, growing from less than two years to an average of more than 3.5 years. A processing time of more than 3.5 years is not reasonable, particularly given that DPR processed these same applications in less than two years in 2019. Its average time for processing other types of applications also increased from 112 days in 2019 to 193 days in 2023. Further, the expanding variability in DPR’s processing time frames makes it increasingly challenging for registrants to predict how long DPR will take to process an application.
- DPR has a decades-old regulation that establishes two standards for the length of time the department may take to evaluate registration data. DPR believes that both of these standards are outdated, but it has not taken action to update them, even though state law requires the department to review its regulations every five years. As a result, DPR is unable to demonstrate that it meets its statutory obligation to register pesticides in a timely manner.
- DPR’s current registration tracking system is unable to track the length of time each evaluation station takes to process certain applications. It also lacks sufficiently reliable data for us to confirm that it processes registration renewals in a timely manner.
- DPR’s lengthy processing of pesticide registration applications can delay consumers’ use of new products. Its lack of timeliness also has financial ramifications for registrants by delaying their ability to sell products in California and potentially losing significant sales.
DPR’s Pesticide Registration Processing Times Have Increased Significantly Since the Pandemic
State law requires DPR to conduct a thorough and timely evaluation before registering a substance as a pesticide for the first time in California.3 However, as Table 1 shows, DPR’s average processing time for new active ingredient and major new use applications increased from 632 days in 2019 to 1,249 days in 2020. By 2023 the amount of time to process these applications had reached 1,345 days on average. Appendix A details additional data on specific application types. New active ingredient and major new use applications—which the text box defines—take extra time for scientific evaluation. For example, DPR requires registrants to submit more data for pesticides with new active ingredients than for other types. Nonetheless, a processing time of more than 3.5 years is not reasonable, particularly given that DPR processed these same applications in 2019 with an average processing time of less than two years.
Categories of Registration Applications
New Active Ingredient: An application to register a new pesticide product containing a new active ingredient not currently registered in California, or an application to amend a product to include such an ingredient.
Major New Use: An application to register a new pesticide product containing an active ingredient found in currently registered pesticide products and proposing a new agricultural, aquatic or other specified use for the first time; or an application to amend a product to include such a use.
Other Application Types
Label Amendments: An application to make certain changes to a product’s label or formulation, such as including additional use sites, crops, and pests or to adjust the stated percent of an active ingredient after registration. This category excludes amendments involving a new active ingredient or major new use.
Currently Registered Active Ingredients: An application to register a new pesticide product containing active ingredients currently registered in California.
California-Only Products: An application to register a pesticide product that is not required to be registered by the U.S. EPA.
Special Local Needs and Experimental Use Permits: An application to register or amend a pesticide product to address a special local need or to request an experimental use permit.
Source: DPR’s Pesticide Registration Process Desk Manual and internal policy.
New active ingredient and major new use applications represent only about 1 percent of the registration applications DPR receives each year. Nonetheless, its average processing time for other application types has also steadily increased since 2019. Specifically, in 2019 DPR averaged 112 days to process other applications: by 2023 this time frame had grown to 193 days. More than half of these other types of applications were for label amendments, which the text box describes.
During these same five years, application processing times also became more variable, creating uncertainty about timing for businesses wanting to sell pesticides in California. For new active ingredient and major new use applications, variability doubled from 2019 to 2023, as Table 1 shows. Variability has also increased for other applications since 2019. The expanding variability in DPR’s processing time frames make it increasingly challenging for registrants to predict how long an application will take DPR to process.
The length and variability of DPR’s processing time frames indicate that it receives more applications than it can effectively and efficiently handle. During the five‑year period, DPR received 469 more applications than it processed, which DPR has indicated creates backlogs that increase registration processing delays. As of January 1, 2024, DPR had 1,606 open applications still in review.
One of the primary barriers to DPR’s efficient and predictable processing of applications is the time necessary for the applications to be assessed at the necessary evaluation stations. As we explain in the Introduction, DPR has 10 such stations that may evaluate a particular application. DPR submits most applications to these stations sequentially, rather than to all pertinent stations at the same time. The only time it performs simultaneous evaluations is for new active ingredient and major new use applications, unless otherwise approved by the branch chief. Because of the sequential nature of most of DPR’s reviews, delays at one or more stations can have a significant impact on the processing time for an application.
Although some of the 10 stations report not having backlogs of applications, other stations report having extensive backlogs, requiring the applications to sit in a queue for months. For example, the Human Health Assessment branch chief stated that because it is understaffed, the Human Health Assessment station typically takes up to two months to begin an evaluation. DPR also explained that its Ecotoxicology station has a longstanding and lengthy backlog, which has contributed to significant delays in the overall processing time. A branch chief said that in one particular case, an application sat in the queue in the Ecotoxicology station for one year before being evaluated.
In addition, the length of DPR’s evaluations varied by station. Table 2 shows the average number of days each evaluation station took to process other applications during our audit period.4 The number of applications that stations received does not necessarily correlate to the processing times. In fact, the Chemistry, Human Health Assessment, Microbiology, and Pest and Disease stations received the most applications, yet four other stations took longer to process fewer applications. Notably, the evaluation times in all stations varied substantially, further demonstrating the challenge registrants experience in predicting how long DPR will take to process their applications. The variation in time frames likely reflects that a number of factors can affect how long an application spends at a station, including the depth of scientific review or the need to collect additional documentation from a registrant.
Regardless of backlogs and external factors, one branch chief explained that scientific evaluations take time to complete because of the necessary complexities of the review process. Depending on the pesticide product, the application may require formal scientific review at one or more evaluation stations. For example, the evaluation scientists in the Chemistry station evaluate product chemistry (except for microbial products), environmental fate, and residue chemistry data. During scientific review, Chemistry evaluation scientists examine the product’s ingredients and the tests conducted to determine, in part, its ability to persist and move through the environment. The Chemistry station uses thresholds outlined in regulation to determine whether the active ingredient has the potential to leach into groundwater. If it exceeds these thresholds, the Chemistry station routes the application to the Groundwater station where scientists further evaluate the potential for groundwater contamination.
Similarly, in addition to their other evaluation responsibilities, the Plant Physiology station determines whether to route an application to the Air station. Each station reviews the pesticide product’s data following federal and state requirements in order to protect California’s environment and inhabitants. The variability in the number of stations and time spent in each station makes predicting DPR’s processing times difficult. The Evaluation Branch chief explained that the rigorous evaluation process is a necessary component that takes time to complete.
We reviewed a selection of 20 applications with above-average and close-to-average registration time frames to identify causes and effects of specific delays. Although the application files we reviewed did not always include clear explanations for delays, interviews with managers revealed that 13 of the 20 applications sat in a queue waiting for an evaluation to begin, and 12 of the 20 applications required the receipt of additional data from the registrant. For example, the Evaluation Branch chief indicated that one application sat in the queue for the Ecotoxicology station for more than two years before evaluation began. The evaluation took about four months to complete after Ecotoxicology began its work.
The Evaluation Branch chief stated that understaffing—an issue we discuss later in this report—is the main cause of these evaluation station backlogs. Waiting for a registrant to submit requested information also contributed to delays in more than half of the applications we selected. The Evaluation Branch chief explained that, in one instance, Ecotoxicology staff waited eight months for a registrant to submit necessary documentation. Although we reviewed only a small fraction of the total applications DPR receives, these same factors may have contributed to delays in other instances.
DPR Has Not Taken Steps to Update Timeliness Standards That It Considers Outdated
We identified two different standards in DPR’s regulations that specify the amount of time the department may take to complete its evaluations of data for pesticide registrations and amendments; however, DPR believes that these standards are outdated. The regulation states that DPR must complete the evaluation of data submitted for a pesticide containing any active ingredient not currently registered with DPR or for any new major use within 120 days of receipt of all such data, and within 60 days of receipt of such data for all other pesticides. DPR has not substantively amended this regulation since 1989, even though it believes that these time frames are no longer relevant given modern scientific review requirements.
According to DPR’s chief deputy director, the department has not sought to update the timeliness standards in regulation because of the complexity of doing so, coupled with ongoing developments for a new electronic registration submission system that we discuss in the next section. She also noted that upcoming process improvements and the hiring of additional staff will change how fast DPR can process registration applications. Finally she stated that existing backlogs make it difficult to establish predictable timelines. However, DPR’s regulations require it to review its pesticide regulations, including the regulation that establishes the timeliness standards, every five years, so it is imperative that these timelines be amended to include an updated and currently valid timeliness standard.
Regardless of whether the existing standards are outdated, DPR’s current method of tracking registrations does not capture the appropriate information to determine whether it is meeting those timeliness standards. The current standards focus on the length of time DPR takes to evaluate the necessary data once it has received it. However, DPR’s current tracking system measures overall processing times and the processing times of stations, both of which include the time DPR spends waiting for registrants to provide it with data. DPR does not measure processing times once an application is complete and it has received the required data. Thus, DPR is not able to accurately monitor and report the length of its data evaluation process.
Although DPR annually publishes a notice that includes the pesticide registration program’s annual processing timelines, DPR does not identify a timeliness goal or target in that notice. Addressed to pesticide registrants and other stakeholders, the notice states that its purpose is to improve transparency, that the reported numbers reflect DPR’s average completion time for applications for each of the past five years, and that these numbers may be used to estimate the potential time frames for future submitted applications. However, adherence to a valid and measureable standard could help inform DPR’s efforts to measure the registration program’s staffing levels and its planned efficiency improvements. Further, while DPR is waiting for its major changes to take effect, it should monitor and publicly report on its efforts to reduce backlogs and registration time frames.
As of June 19, 2024, the Legislature is considering Assembly Bill 2113 (AB 2113), which would require DPR to complete pesticide registrations within specified time frames and publicly report its average processing times, among other requirements. Specifically, AB 2113 would require DPR to register or amend a pesticide product within six months or, if the product contains a new active ingredient, within two years, beginning no more than 15 business days following its receipt of the application. These time frames would be paused in certain circumstances, such as when the registrant fails to correct application deficiencies and when a product revision requires U.S. EPA approval. AB 2113 would also require DPR to annually report on its website the proportion of applications it completed within these time frames and the average number of days that applications spent in intake and at each scientific evaluation station. If this version of AB 2113 is enacted, these time frame and reporting requirements would commence in 2028. However, AB 2113 may be amended by the Legislature as it continues through the legislative process.
Limitations of DPR’s Current Registration Tracking System Reduce the Transparency of Registration Delays
DPR’s current registration tracking system lacks the sophistication to track the lengthiest types of registrations at individual evaluation stations. As we show in Table 1, its major new use and new active ingredient registration evaluations took more than 1,000 days on average from 2019 through 2023. However, its tracking system does not record the time these applications spent at specific evaluation stations because the evaluation stations review these applications simultaneously. According to one of DPR’s research data specialists, the department did not design its tracking system to capture the evaluation times of applications while they are reviewed simultaneously. Given the lengthy time frames for reviewing major new use and new active ingredient applications, DPR should be aware of how long it takes each station to process these registrations so that it can accurately understand each station’s staffing needs. For example, if DPR tracked processing times by station for these applications, it could identify which stations may contribute the most to delays and then further research the reasons why.
Additionally, DPR lacks sufficiently reliable data for us to confirm that it processes registration renewal applications in a timely manner. State regulation generally requires DPR to issue renewals within 60 days of its receipt of an accurate and complete application, and the data it provided indicated that it generally does so. However, we identified problems with the source documentation supporting the data which made us question its reliability. Specifically, although the renewal data were complete, DPR’s inconsistent processes and supporting documentation affected our ability to test its accuracy. According to a manager in the registration branch, the branch does not have formal policies and procedures for processing renewal applications, resulting in incorrect dates in the data or missing dates in the source documentation. Its weak processes prohibit DPR from reliably demonstrating that it is processing renewals in accordance with the standards in the regulations. Further, the registration manager noted that the branch had not analyzed the renewal data to determine the timeliness of the renewal process because staff processed renewals quickly, and DPR had not received renewal timeline questions or concerns.
DPR’s current registration tracking database also lacks the ability to determine whether its evaluation times differ between products with different use types, which the audit request asked us to evaluate. DPR’s paper application form includes six types of pesticide uses that a registrant can select, which we include in the text box. DPR’s tracking database does not currently store information about the type of use of registered pesticide products. Our review of 20 applications did not identify the evaluations of any particular use types as consistently more lengthy than others. For example, nine applications indicated an agricultural use and the length of their processing ranged from 934 to 2,987 days. Similarly, the seven applications that indicated a household/home garden use had processing times ranging from 712 to 3,321 days.
DPR’s Registration Application Types of Uses
- Agricultural
- Household/Home Garden
- Institutional (hospitals, schools, etc.)
- Industrial End Use
- Structural
- Manufacturing/Reformulation Only
Source: DPR registration application forms.
Although DPR’s current system lacks the ability to determine the timeliness of specific pesticide use types and to track certain application processing times, the department intends to begin implementation of a new system in August 2024 that will improve its ability to track application processing times. DPR’s new and fully integrated information and document management system called the California Pesticide Electronic Submission Tracking system (CalPEST) will track, for example, the processing time of applications routed simultaneously to different evaluation stations and will include pesticide use types in its data. The registration branch chief stated that CalPEST will also automate the registration renewal process, which will include standardizing procedures and supporting documentation. However, to ensure that CalPEST sufficiently resolves the deficiencies we found regarding simultaneous review, renewals, and use types, it is important that DPR demonstrate CalPEST’s ability in these areas. We discuss this new system in detail in a subsequent section of this report.
Lengthy Application Processing Times Can Negatively Affect Consumers, Businesses, and the State
When DPR does not process pesticide registration applications in a timely manner, it can delay the benefits consumers will realize from the use of those pesticide products. Many household products are classified as pesticides, including disinfectants, mosquito repellents, and products that kill mold and mildew. These pesticides can be used for the protection of health and safety in homes and hospitals. Additionally, farmers use pesticides to control pests that damage food and other crops. Because farmers generally cannot use a new pesticide until DPR has approved its registration, a delay in the registration process could cause them to forgo planting a crop altogether because the pesticide would not be available to protect it.
The lengthy registration process can also affect registrants by delaying their ability to sell products in California. This not only has a financial impact on the registrants but also on DPR, which does not collect mill assessments until products are registered and sold. In our review of 20 applications, we looked at each product’s first quarter of reported sales for the seven pesticides that had reported sales. These sales ranged from $200 to more than $34,000. Given that there are tens of thousands of registered pesticides in California, delayed registrations may cause businesses to lose significant sales and DPR to lose the mill assessment revenue associated with those sales.
Finally, delays can cause companies to miss deadlines that stores selling pesticides may set. DPR noted in the feasibility study report for its new registration application tracking system that the market for consumer pesticide products is driven by a registrant’s ability to place products in large stores like Wal-Mart and Home Depot. Such stores accept new products only two times per year. If a registrant misses one of these two deadlines, it has to wait another six months to get its product into the marketplace and start generating revenue from it.
Insufficient Staffing and Inefficient Processes Have Contributed to the Delays in DPR’s Processing of Pesticide Registration Applications
Key Points
- DPR does not have an adequate number of staff to process registration applications in a timely manner and it has not established a formal and ongoing process for determining the number of registration program staff that it requires.
- DPR’s current pesticide registration processes are paper-based and rely on data systems that are not fully integrated, contributing to delays and creating opportunities for the introduction of errors.
- In August 2024, DPR plans to implement a new information and document management system. The long-delayed system, which has a final budget of more than $26 million dollars, should allow DPR to transition from its paper-based registration process to a more efficient electronic one, among other benefits.
- DPR has recently undertaken initiatives that could improve its registration process, but it is too soon to determine their effectiveness.
DPR Lacks Sufficient Staff to Process Registration Applications in a Timely Manner
DPR’s consistent delays in processing registrations indicate that it does not have an adequate number of staff to carry out its registration duties within a reasonable time frame. DPR has processed fewer pesticide registration applications than it receives for the past several years, which DPR has indicated creates backlogs that increase registration processing delays. DPR reported in its 2023 registration program annual notice on processing timelines that it is understaffed relative to its registration workload. It has taken steps to begin requesting additional positions. However, DPR’s chief deputy director has indicated that it does not have a formal or ongoing process in place to determine whether its registration program has appropriate staffing levels before backlogs develop.
To determine the degree to which staff vacancies contributed to DPR’s staffing problems from 2019 through 2023, we assessed DPR’s vacancy rates for positions involved in its registration process. In addition to the Registration Branch, we included the branches that house DPR’s 10 evaluation stations: the Pesticide Evaluation Branch, Human Health Assessment Branch, Environmental Monitoring Branch, and Worker Health and Safety Branch.
Based on DPR’s internal vacancy reports, the approximate vacancy rate of its positions with registration-related duties ranged from 6 percent to 20 percent from 2019 through 2023. As of January 1, 2024, the approximate vacancy rate of these positions was 10 percent. A Legislative Analyst’s Office report published in March 2024 noted that the statewide vacancy rate for California departments has consistently been above 10 percent for the last 20 years and was about 20 percent as of February 2024. This indicates that DPR’s vacancy rate falls within a reasonable range for state agencies—although vacancies may still have affected its ability to process registrations in a timely manner.
Understaffing is a particularly significant problem for the branches that play larger roles in the registration process: the Registration, Evaluation, and Human Health Assessment Branches. For instance, the Registration Branch chief believes that even if it filled all of the branch’s positions, DPR might still need additional regulatory scientists to address delays. As we previously describe, the Human Health Assessment and Evaluation Branches have indicated that their branches have backlogs that can lead to months of delay before staff can begin an evaluation. These backlogs indicate that the stations’ current workloads exceed what the evaluation scientists can effectively process in a timely manner, which is generally consistent with the perspectives of scientists we interviewed.
DPR has taken steps to start resolving its staffing needs. In 2021 and 2022, the Legislature approved funding for DPR to commission an independent study of its mill assessment, which included a workload analysis for the department’s future programmatic needs. That analysis identified 44 additional positions for future programmatic needs in DPR’s registration program. Despite the workload analysis, DPR did not immediately request all 44 additional positions. Rather, in its fiscal year 2023–24 budget change proposal, DPR requested seven additional positions to, among other things, start addressing the needs the analysis identified in the registration program. The Legislature approved funding for the requested seven positions.
For fiscal year 2024–25, DPR has submitted a budget change proposal requesting additional staff. As of June 19, 2024, the Legislature approved a budget bill addressing the proposal, which has not yet been signed by the Governor. DPR indicated that 36 of the requested positions would be funded by the registration fees. Additionally, DPR stated in its request that one of its goals in increasing its staffing levels is to reduce registration backlogs enough to be able to initiate scientific evaluations within 30 days of receiving an application or necessary data. Nonetheless, DPR has acknowledged that it does not have a formal or ongoing process in place to determine whether its registration program has appropriate staffing levels to prevent backlogs from developing. According to the U.S. Government Accountability Office’s (GAO) Handbook for Government Work Force Requirements, determining reasonable staff needs is essential to maintaining and improving an organization’s productivity, including curtailing backlogs in the event of understaffing. Further, DPR must continuously update and link its staffing needs to its performance goals, which demands that it establish a formal procedure for determining the staffing needs of its registration program on an ongoing basis. Given the implications of the impact on DPR, registrants, and consumers when registrations are delayed, it is imperative that DPR develop a process to regularly monitor and reassess its staffing levels specific to registration. A formal process for doing so will enable DPR to take proactive measures that help prevent future backlogs from occurring.
DPR Currently Uses Inefficient Processes to Review Applications
DPR’s regulations require registrants to submit their registration applications by mail, which DPR interprets as requiring them to submit paper applications. Consequently, it currently accepts only paper pesticide registration applications that require manual and labor-intensive processing. DPR receives several thousand registration application packages each year, with the supporting documents for a single application often involving hundreds of pages of research and data. According to DPR, tracking, managing, and storing this large volume of paper documents is cumbersome and time‑consuming. Further, it stated that as the documents move through the registration process, they can be lost or misplaced, resulting in additional processing delays.
Because the applications are paper, DPR’s evaluation stations review most registration applications sequentially, rather than simultaneously, as we previously describe. DPR’s Registration Branch chief stated that making, tracking, and storing physical copies to allow for concurrent reviews of all applications would be time- and cost-prohibitive. Consequently, it does so only for its new active ingredient and new major use applications, which comprise about 1 percent of all applications it receives. For its other applications, DPR maintains the application and supporting documentation as a single set of hard copy documents.
Another inefficiency of DPR’s current registration process is that it relies on data systems that are not fully integrated. DPR uses 24 separate and disparate tracking systems and databases to log, index, manage, and track its product registration work. These include systems for tracking registration submissions, workloads, and required reporting; storing information on all chemicals in or relative to pesticide products; and indexing information on studies submitted in California in support of pesticide product registrations. Although these systems may share some data, not all data interactions are automated, so staff must sometimes manually enter data from one system into others. As a result, DPR risks creating duplicate records and introducing inaccuracies in its data systems. Figure 3 depicts an example of the inefficiencies caused by the current system’s lack of integration.
Figure 3
DPR’s Current Data Systems Result in Inefficient Data Processing

Source: DPR documents and interviews.
Figure 3 shows an example of the inefficient data processing practices within DPR’s current data systems. It is labeled at the top with the words “Tracking System”. Beneath that is the sentence reading “DPR’s Registration Tracking System (tracking system) contains similar data to three other systems, but data updates in one system are not consistently pushed to all related systems. Below that is an image of a data server connected to an image of a folder representing application data in the tracking system, with three data elements, firm name, chemical name, and product name, labeled on the folder. There is a read box between the data server image and the application folder image reading “Because not all data entries and updates are automated, DPR creates duplicate records and risks introducing inaccuracies into its data systems. One data element in the tracking system—the chemical name—is linked to an image of a database labeled the Master Chemical System with a solid green line and appears on the right side of the figure. A legend labeled “Data Link Description” states that the solid green line means that changes to linked data in one system are automatically reflected in other systems. Beneath the image of the Master Chemical System is the statement “Updates to chemical names are automated.”
A second data element in the tracking system, the firm name, is linked to another image of a database labeled Licensing System by a dotted green line, and appears on the left side of the figure. The legend stated that this line indicates linked data from one system are reflected in other systems, but subsequent changes to one system are not automatically reflected in others. These changes must be manually made in each affected system. Beneath the image of the Licensing System is the statement “DPR must manually enter firm name changes into the tracking system.
The third and final data element displayed in the figure, product name, is linked to a third image of a database labeled Product/Label System with a broken red line. The legend states that this broken red line indicates the data are not linked. Beneath the image of the Product/Label System is a statement that “DPR must separately enter product data, such as the product’s name, into both the tracking system and the Product/Label System.
Finally, DPR’s current data systems lack support for some workflow improvements. For example, DPR currently cannot accept electronic payment of registration fees. Instead, it requires registrants to submit paper checks with their registration or renewal applications. DPR explained that this process is cumbersome and may delay payment processing by as much as 15 days.
DPR’s Long-Delayed New Data System to Improve Its Business Processes Is Nearing Implementation
In August 2024, DPR plans to begin implementing CalPEST, a new and fully integrated information and document management system. DPR plans for CalPEST to transition the department from its paper-based, manual registration process to an electronic one; to allow for digital submission of registration and amendment applications; and to allow for concurrent application evaluation. In its original 2015 project feasibility study report for CalPEST, DPR identified several business problems with its current registration tracking system. For example, it recognized that its current registration processes resulted in cumbersome processing, bottlenecks, and inefficiencies, and that registrants submitted incomplete registration and label amendment submissions. It also found that its disparate, stand-alone systems limited its visibility into workload per station and staff and that no single data source existed to register products. It designed CalPEST to address these problems. Nonetheless, it is important to note that although CalPEST will facilitate communication and the movement of information, it will not change the nature of the reviews that DPR’s scientists perform while evaluating registrants’ pesticide applications, which can take several months.
CalPEST’s timeline has increased from what DPR originally envisioned. In 2015 DPR described a planned implementation by June 2017. As Figure 4 shows, DPR extended its schedule for CalPEST three separate times and that the majority of the delays occurred before the final selection of the system integrator, which was the project’s primary contractor. For example, in its first special project report (SPR), approved by the California Department of Technology (CDT) in 2018, DPR cited delays related to unanticipated project oversight requirements and multiple failed vendor procurement attempts caused by vendors submitting proposals that did not meet DPR’s requirements.5 DPR indicated that these factors delayed the project’s implementation by 2.5 years.
Figure 4
Most of the Delays in CalPEST Stemmed From Activities Leading to the Final Selection of a Contracted System Integrator
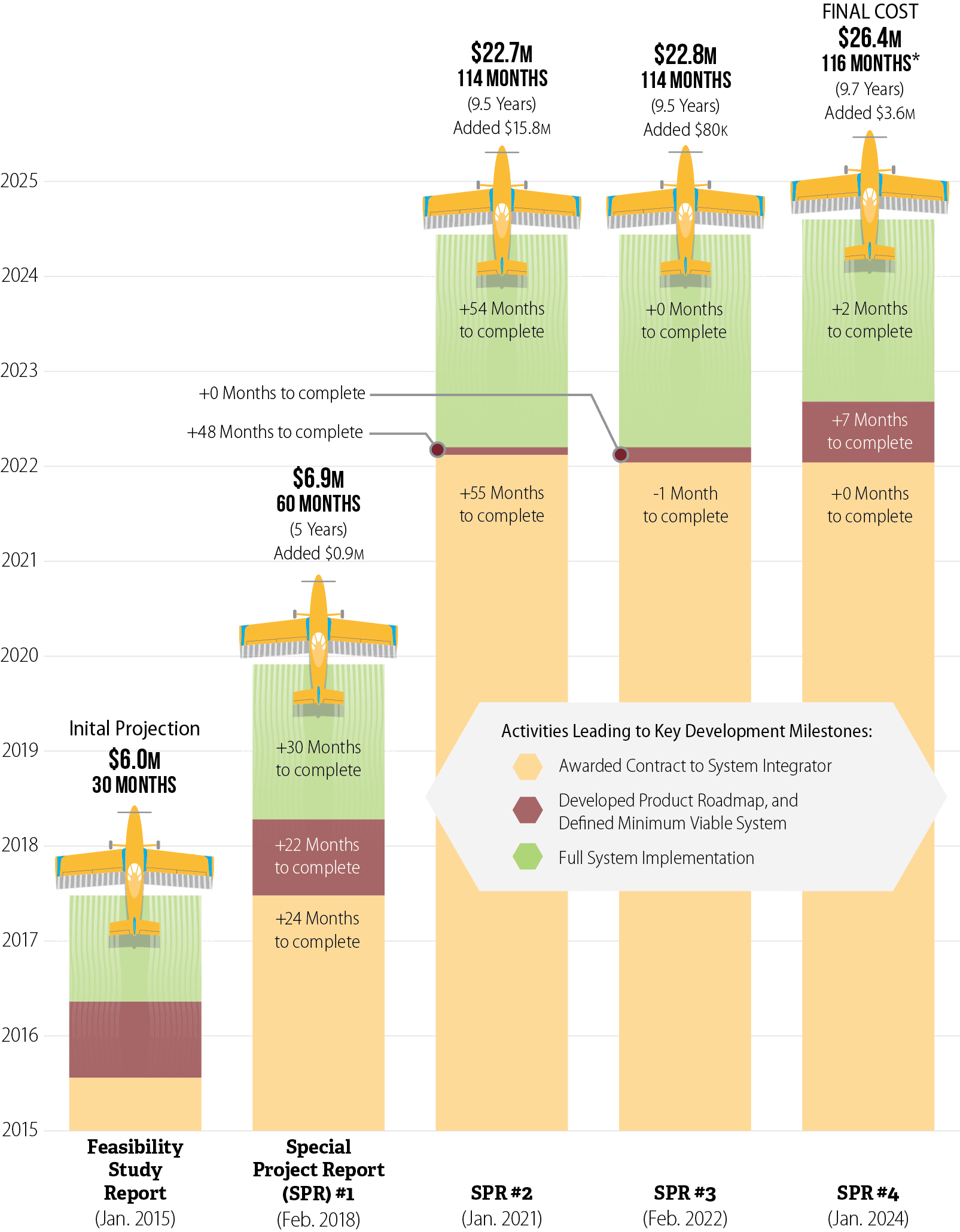
Source: CalPEST Feasibility Study Report and Special Project Reports.
* Beginning in SPR #4, DPR’s implementation of CalPEST is planned in two phases. The first implementation is scheduled for August 2024 and is targeted to provide 80 percent of the system’s functionality, including support for the registration renewal process. This figure reflects the August 2024 implementation date. The second implementation is scheduled for March 2025 and will provide all remaining functionality.
Figure 4 is a bar chart showing increases in schedule and cost for the development of CalPEST through five stages of the project’s development. The figure shows a vertical axis of ten years beginning with 2015 and ending with 2025. It shows five timelines superimposed over those years. Each timeline is divided into three sections, representing activities leading to key development milestones. These three sections are shaded in color tan, brown, and green. A legend explains that the first milestone, in tan, is when DPR was to have “awarded contract to system integrator.” The second, in brown is when DPR was to have developed product roadmap, and defined minimum viable system. The third milestone, in green is full system implementation.
The first bar and timeline shows DPR’s initial projection of CalPEST’s schedule and project costs, as described by its Feasibility Study Report from January 2015. In this timeline, DPR expected to award its system integrator contract in mid-2015. It expected to complete development of its product roadmap and complete its definition of the minimum viable system by early to mid-2016, and expected full system integration by mid-2017. Based on its initial projection, DPR believed it could implement CalPEST in 30 months at a cost of $6 million.
The second bar and timeline shows DPR’s projection of schedule and cost based on its Special Project Report, or SPR, Number 1 from February 2018. In this timeline, DPR indicated that it awarded its system integrator contract in mid-2017, 24 months beyond what it expected in its initial projection. It projected it would complete its development of its product roadmap and definition of the minimum viable system in early 2018, 22 months later than it initially expected, and that it would complete full system integration at the end of 2020, 30 months after the date it described in its initial projection. Under SPR1, DPR expected it would implement CalPEST in 60 months—or five years—at a cost of $6.9 million—a $900,000 increase from its initial projection.
The third bar and timeline shows DPR’s projection of schedule and cost based on its SPR number 2 from January 2021. In this timeline, DPR projected it would award its system integrator contract in early 2022, 55 months later that the date identified in SPR1. It further projected it would also complete its development of its product roadmap and definition of the minimum viable system in early 2022, 48 months after the date described in SPR1, and that it would complete full system integration in mid-2024, 54 months after the date it described in SPR1. Under SPR2, DPR expected it would implement CalPEST in 114 months—or 9.5 years—at a cost of $22.7 million—a $15.8 million increase from SPR1.
The fourth bar and timeline shows DPR’s projection of schedule and cost based on its SPR number 3 from February 2022. In this timeline, DPR projected it would award its system integrator contract at the beginning of 2022, one month earlier than it projected in SPR2. It further did not change its projections for completing development of its product roadmap and definition of minimum viable system, or full system integration from those it identified in SPR2. Under SPR3, DPR expected it would implement CalPEST in 114 months—or 9.5 years—at a cost of $22.8 million—an $80,000 increase from SPR2.
The fifth and final timeline shows DPR’s projections of schedule and cost based on its SPR number 4 from January 2024. In this timeline, DPR reported it awarded its system integrator contract at the beginning of 2022, as it expected in SPR3, and that it completed development of its product roadmap and defined its minimum viable system mid-2022, seven month later than its SPR3 projection. It finally projected full system implementation in mid to late 2024, 2 months after the date described in SPR3. Under SPR4, DPR expects to implement CalPEST in 116 months—or 9.7 years—at a cost of $26.4 million—a $3.6 million increase from SPR3.
An asterisk attached to the 116 months for SPR #4 is explained at the bottom of the figure with a statement that “beginning in SPR #4, DPR’s implementation of CalPEST is planned in two phases. The first implementation is scheduled for August 2024 and is targeted to provide 80 percent of the system’s functionality, including support for the registration renewal process. This figure reflects the August 2024 implementation date. The second implementation is scheduled for March 2025 and will provide all remaining functionality.”
In its second SPR approved in 2021, DPR identified further delays related to ending its original system integrator contract and to tasks it undertook in re-planning the project, including conducting additional market research and refining the existing requirements. This added an additional 4.5 years to CalPEST’s implementation schedule. The last two SPRs, from 2022 and 2024, added only two months to the project’s timeline. In total, 6.5 of the 7.2 years of delays in the development of CalPEST are tied to activities that occurred before DPR’s awarding of the final system integrator contract.
The project’s costs have similarly increased: DPR’s 2018 SPR added nearly $900,000 to the project’s original estimate of $6 million. It identified this funding as necessary for increased staffing and project oversight costs, as well as post-implementation support costs that it inadvertently left out of the original budget. DPR’s 2021 SPR added another $15.8 million to the project for additional system integrator vendor costs, project management and support services costs, ongoing project staffing costs, and software maintenance agreements. In its 2022 SPR, DPR added just over $80,000 for maintenance and operations expenses and increasing staffing costs, and in its 2024 SPR, it added $3.6 million for hardware and software purchases and contract service extensions. In total, the project’s projected cost grew from $6 million to $26.4 million during its nine years of development.
As of its 2024 SPR, DPR expects to begin implementation of CalPEST in August 2024 and finish implementation by March 2025. It does not expect further changes to the project’s scope, costs or timeline.6 Further, DPR’s project status reports, which convey the overall status and progress of an IT project to CDT, and CDT’s independent project oversight reports, which identify and quantify issues and risks affecting project objectives, noted that the project was on track to satisfy its business objectives within the approved time frame and budget. In addition, the contractor conducting the project’s independent verification and validation process, which ensures that a product, service, or system meets requirements and specifications and that it fulfills its intended purpose, has identified issues related to the development of CalPEST, and tracked progress toward the resolution of those issues. As of February 2024, DPR has resolved the majority of the issues identified. In October 2022, DPR commenced user acceptance testing, which will continue until CalPEST is fully implemented in March 2025. Its Registration Branch chief reported that the testing so far has not resulted in any major concerns with the development of CalPEST. However, despite those indications, CalPEST’s history of delays and budget changes demonstrate the risk that the project budget or timeline could change again.
DPR Has Recently Undertaken Additional Initiatives That May Improve Its Efficiency
In addition to the development of CalPEST, DPR has recently undertaken workload and policy initiatives to improve the efficiency of its business processes. For example, historically, DPR assigned a regulatory scientist to each company that registered pesticides products in California. That regulatory scientist reviewed a company’s new product registration and product amendment applications and served as DPR’s liaison with the company throughout the registration process. However, in September 2023, DPR began a process to shift assigning applications to one of four teams of regulatory scientists, rather than assigning each application to an individual scientist. Further, in February 2024, DPR commenced assigning these applications to the teams based on the pesticide’s active ingredient instead of based on the company.
DPR identified multiple benefits from this change. For example, it explained that it should result in more consistent application reviews and shorter processing time frames. It also stated that the change would increase awareness and understanding of data requirements and labeling issues specific to active ingredients among team members and supervisors. Further, it indicated that this change would provide more efficient tracking of federal decisions that DPR considers when reviewing pesticides, including mitigation required for products containing specific active ingredients.
As of late April 2024, DPR had not yet performed a formal evaluation of its new workload assignment process, noting that the process was still fairly new. The Registration Branch chief explained that, because of its existing backlog, it had not been able to fully implement and evaluate the new workload distribution. However, after it has fully implemented the change, he expects to be able to operate in a more efficient manner.
In January 2024, DPR implemented another policy that could help expedite application processing times. State law requires that applications to register or amend a pesticide product in California include required data or reference data previously submitted. DPR’s new policy provides that if during the scientific evaluation an evaluation station determines that the supporting documentation is incomplete, DPR will email the registrant to inform them of the deficiencies and provide them with 15-business days to provide the required supporting documents. If the registrant has not responded within that time frame, DPR will conclude the data do not support registration or amendment of the product for that specific evaluation station, and the product will then proceed to the next evaluation station for review. DPR’s policy indicates that if DPR receives the missing documentation while the product is still under scientific evaluation by any other station, the product can be routed back to the evaluation station where the deficiency was identified. After all evaluation stations have completed their reviews, DPR will make its determination to register or deny the application. In this way, DPR should, over time, see a reduction in delays in evaluating applications caused by missing data necessary at one evaluation station by moving the application on to the next evaluation station. However, because of its recent implementation, it is too soon for DPR to determine whether it has gained efficiencies from this change.
DPR is Making Efforts to Raise Fees to Address Its Increasing Expenses
Key Points
- DPR’s program expenses have increased in recent years. As a result, DPR is proposing to raise registration fees and the mill assessment to support increased staffing and employee compensation.
- DPR has mechanisms in place to ensure the appropriate collection of its mill assessment, but it should improve its processes by creating a formal, documented methodology for selecting the companies it audits.
Addressing Its Staffing Needs and the Inefficiencies in Its Registration Application Processes Is Raising DPR’s Expenses
As we discuss in the previous sections, DPR’s lengthy and variable registration time frames can be attributed, at least in part, to its lack of adequate staffing and to inefficiencies in its registration process. DPR is currently working to address both of these problems; however, doing so is increasing its costs. To address these increases in costs, DPR is requesting legislative changes to increase its mill assessment and is proposing regulatory changes to increase its registration fees. Mill assessment and registration fee revenue collectively accounted for 95 percent of the DPR Fund’s fiscal year 2022–23 revenue.
DPR has already raised registration fees in recent years. DPR’s accounting records show that in fiscal year 2019–20, registration program expenses exceeded revenues by nearly $300,000. In 2021 it increased its registration renewal fees for each pesticide product from $1,150 to $1,525. At the time, DPR projected that this $375 increase would be sufficient to cover expenses through fiscal year 2025–26. However, the department is now reporting in a notice addressed to pesticide registrants and other stakeholders that it has incurred about $4 million in unanticipated registration expenditures for statewide employee compensation increases and retirement adjustments and $1.2 million per year to support the additional positions authorized by the Legislature in 2023. DPR projects that the registration program’s deficit will continue to grow in the coming years. DPR’s accounting records show that in fiscal year 2022–23, the registration program costs exceeded revenues by more than $800,000. Consequently, as of April 2024, DPR was proposing regulatory changes to raise its registration renewal fee by $775—from $1,525 to $2,300—and it is also planning on increasing other registration fees, including new product application fees.
DPR is authorized to expend registration fee revenues only for the purposes of carrying out its registration program. However, state law makes mill assessment revenue generally available to support DPR’s operations, including the registration program if the registration fees are insufficient to cover the program’s costs. However, because of DPR’s pending proposals that could raise both its registration fees and staffing costs in the coming year, it is unclear whether DPR’s registration program would need to rely on mill assessment revenue in the future.
Most of the programs that DPR pays for with the mill assessment have also faced increasing costs in recent years. As the text box shows, eight of DPR’s 10 programs are primarily funded by mill assessment revenue. DPR’s accounting records show that since fiscal year 2018–19, the cost of the programs supported by the mill assessment have increased overall by more than $18 million. DPR attributes the increasing costs to additional requirements the department must meet and to increasing personnel costs associated with new positions and raises negotiated by bargaining units.
DPR’s Programs and Funding Sources
Eight of DPR’s 10 programs are funded primarily by the mill assessment:
- Human Health and Environmental Assessments
- Pesticide Use Reporting
- Monitoring and Surveillance
- Mitigation of Human Health Risk
- Mitigation of Environmental Hazard
- Pest Management
- Enforcement
- Mill Assessment
The Pesticide Registration and Licensing and Certification programs are funded primarily by registration and licensing fees, respectively.
Source: DPR accounting records, budget documents, and staff interviews.
The Legislature last raised the cap on the mill assessment for transactions commencing in 2004. According to one of DPR’s budget change proposals, this level of mill assessment—2.1 cents per dollar of sales—has not kept pace with the expansion of essential programs and legislative mandates. In 2021 the Governor proposed replacing the flat-rate mill assessment with a risk-based tiered rate. Although the Legislature declined this proposal, the Legislature provided funding for DPR to commission a study to identify future options for a tiered mill assessment. The mill assessment study recommended a flat rate increase that DPR should phase in over several years. In January 2024, the Governor released his fiscal year 2024–25 state budget proposal, which includes a proposed increase in the mill assessment to almost 2.9 cents per dollar of pesticide product sales, phased in over three years, and with a statutory cap of almost 3.4 cents per dollar thereafter. DPR projects that this increase, if approved by the Legislature, will support its programmatic needs through fiscal year 2028–29. As of June 19, 2024, the Legislature is considering AB 2113, which we previously describe, to require similar increases to the mill assessment that would begin in July 2024 and continue until June 2027. Because DPR’s expenses and revenues may change, it is unclear whether there will be excess mill assessment revenue available to help cover future registration program needs, if necessary.
Analysis of the total amounts in the DPR Fund also shows that DPR’s revenues and expenses have increased in recent years. The DPR Fund receives revenue from three main sources: mill assessments, registration fees, and licensing and certification fees. Figure 5 shows that since fiscal year 2018–19, DPR’s overall revenues and expenses have increased by around $20 million. DPR’s expenses increased significantly in fiscal year 2021–22, which DPR reports is partially due to costs related to implementing CalPEST. DPR’s revenues have also steadily increased over the past five years.
Figure 5
In Recent Years, the DPR Fund’s Revenues and Expenses Have Increased While Its Balance Has Decreased
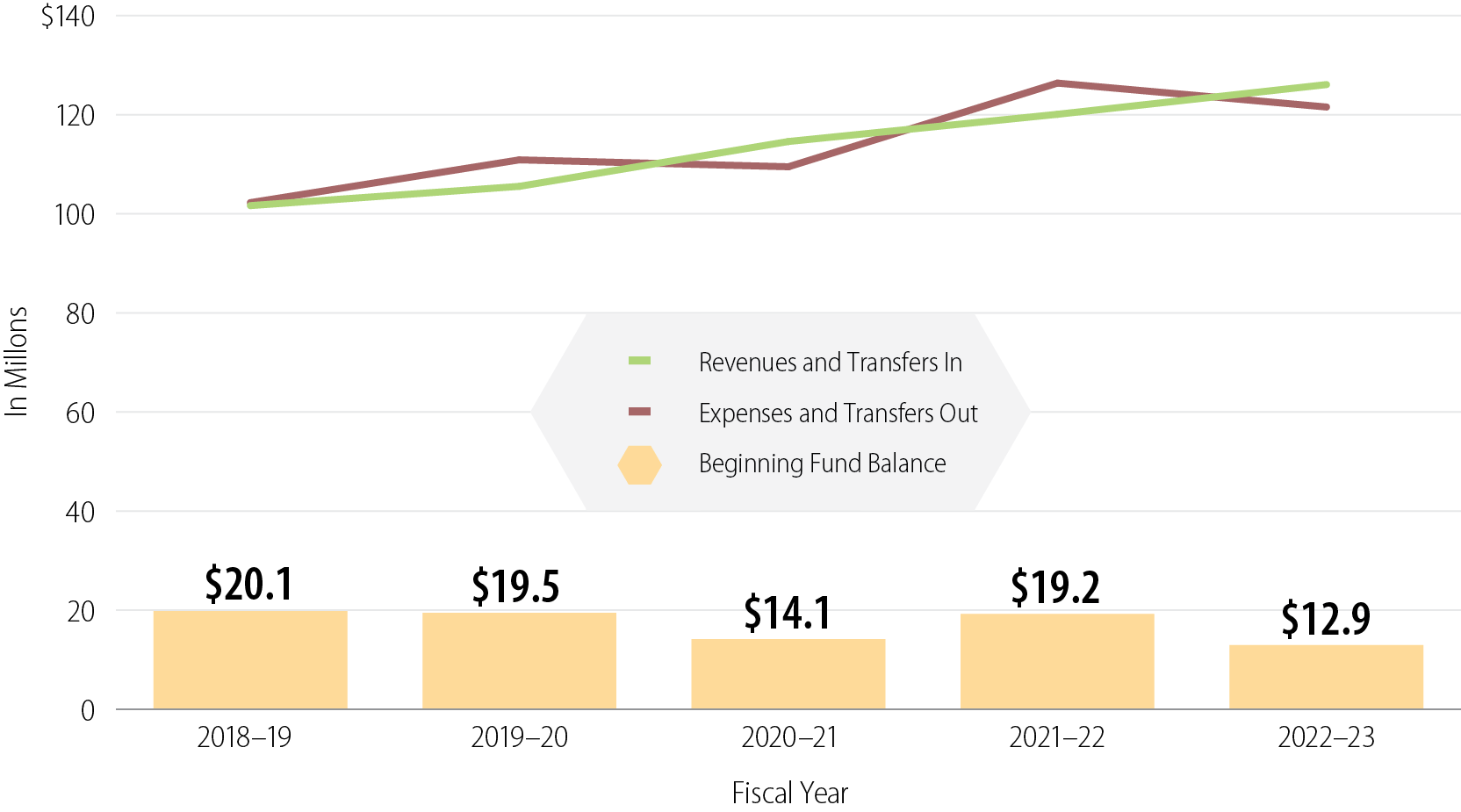
Source: DPR’s accounting records.
Note: The DPR Fund receives revenue from three main sources: mill assessment revenue, registration fees, and licensing and certification fees.
Figure 5 is a graph that shows the DPR Fund’s Revenues, Expenses, and Fund Balance for fiscal years 2018-19 through 2022-23. The graph has a red line, a green line, and yellow bars toward the bottom of the graph. The vertical axis is labeled “In Millions”, and goes from $0 to $140 in increments of 20. The horizontal axis is labeled “Fiscal Year” and lists fiscal years 2018-19, 2019-20, 2020-21, 2021-22, and 2022-23. The green line is labeled “Revenues and Transfers In.” This line is at $101,650,078 for 2018-19, $105,537,375 for 2019-20, $114,586,082 for 2020-21, $120,077,920 for 2021-22, and $126,072,764 for 2022-23. The red line is labeled “Expenses and Transfers Out.” This line is at $102,250,546 for 2018-19, $110,883,366 for 2019-20, $109,487,997 for 2020-21, $126,374,666 for 2021-22, and $121,546,314 for 2022-23. The yellow bars are labeled “Beginning Fund Balance.” These bars show $20.1 million for 2018-19, $19.5 million for 2019-20, $14.1 million for 2020-21, $19.2 million for 2021-22, and $12.9 million for 2022-23.
DPR’s increasing expenses have caused the DPR Fund’s balance to decrease in recent years. Figure 5 demonstrates that the DPR Fund’s balance has decreased from $20 million in fiscal year 2018–2019 to about $13 million in fiscal year 2022–23. DPR’s chief deputy director noted that DPR does not have an official position on what a prudent reserve should be; however, the Government Finance Officers Association recommends that a government agency’s fund balance should, at a minimum, equal at least two months of regular operating revenues or expenditures. Two months of DPR’s operating expenses in fiscal year 2022–23 would equal a fund balance of about $20 million. However, increasing operating costs threaten the stability of the fund’s already shrinking reserve. If increases from the Governor’s Budget for the mill assessment are approved, DPR projects that its fund balance will reach more than $40 million by fiscal year 2028–29.
DPR Uses Two Primary Methods to Track Pesticide Sales and Ensure Proper Payment of Its Mill Assessment
State law requires pesticide brokers, pest control dealers, and registrants to report quarterly the value and quantity of pesticides they sell into or within California that are subject to the mill assessment. DPR’s senior management auditor indicated that staff send reminders of upcoming reports due to companies that are required to submit quarterly reports and delinquency notices for reports not received. DPR staff also validate the data from the quarterly reports DPR receives by recalculating the reported sales totals as they enter the data into the department’s database. To ensure that these companies are properly reporting those sales and paying the required mill assessment, DPR also verifies submitted sales data by conducting audits of a selection of the companies. In fiscal year 2021–22, it conducted 35 audits across 12 states, and in fiscal year 2022–23—the most recent completed fiscal year—DPR conducted 46 audits across 13 states. Because it lists penalties that may result from these audits on its website, DPR’s audits can act as a deterrent to registrants’ nonpayment of the mill assessment.
DPR’s audit steps are reasonably designed to detect nonpayment of the mill assessment. For example, one of the standardized steps in the audit program is to review and document at least on a sample basis the reports, schedules, and records the auditee used in calculating its mill assessment. It also details a step for the auditor to determine whether the methodology used captures all eligible sales and products subject to the mill assessment. In each of the audits conducted in fiscal years 2021–22 and 2022–23 that we reviewed, we observed that DPR consistently performed the same key tasks, such as reviewing the auditee’s background and historic sales information, walking through the auditee’s sales transaction cycle, reviewing the auditee’s calculation of mill assessment, examining a selection of invoices, and reviewing the auditee’s sales catalog for unregistered products.
However, we noted that DPR lacks a formal policy for selecting which companies it will audit each year. According to DPR, it considers multiple factors when selecting companies to audit, including a company’s sales volume, changes to its sales volume, its geographic location, and complaints or tips DPR may have received. Although these methods are reasonable, DPR lacks a formal, documented policy for ensuring that its selection will consistently identify those companies with the greatest risk of misreporting sales data or that the audits act as a deterrent to nonpayment for all pesticide companies. State law requires state agencies to establish processes, including policies and procedures, that provide reasonable assurance that the agency can meet its objectives. However, DPR could not provide a formal policy or desk manual describing its methodology for selecting companies for its annual audits. DPR indicated that it was not opposed to creating such formal guidance, but has not done so yet.
DPR also conducts marketplace surveillance inspections (inspections) to ensure that pesticides sold in California are properly labeled and registered, initiating 174 inspections in fiscal year 2022–23. DPR’s inspections are guided by federal and state inspection manuals, which have clear methodologies for carrying out inspections. These manuals provide guidance on identifying appropriate places to inspect, such as hardware stores, medical and dental suppliers, or janitorial supply dealers; reviewing pesticide products; and collecting shipping records. The state inspection manual also describes how to prepare for the inspection, to establish priorities when conducting an inspection, and to identify the necessary steps after finding a violation of the law.
DPR’s inspections and audits have identified violations of the legal requirement that only registered and properly labeled pesticides may be sold in California and have resulted in the collection of penalties that may act as a deterrent to violating state and federal laws. For example, DPR issued a violation to a company selling a floor polish designed to inhibit the growth of bacteria, mold, and mildew. DPR found that the company had not registered the product for sale in California, and it levied a civil penalty of $5,500 on the company. The text box lists the number of companies to which DPR issued violations in the past several years and the total amounts of the associated penalties. In addition, DPR identifies on its public website the companies to which it issues violations and the amount of the assessed fines and penalties and this, too, may act as a deterrent to improper activities for other companies.
DPR Levied Fines and Penalties to Pesticide Companies
Fiscal Year 2021–22: 36 companies totaling $808,000
Fiscal Year 2022–23: 43 companies totaling $2.25 million
Fiscal Year 2023–24 (Quarters 1 and 2): 25 companies totaling $254,000
Source: DPR’s public reports of fines and settlements.
Other Areas We Reviewed
To address the audit objectives approved by the Joint Legislative Audit Committee (Audit Committee), we reviewed DPR’s training requirements for staff involved in the registration process and recent changes to DPR’s label amendment process.
DPR’s Training Requirements for Scientists
DPR uses two main types of staff during the registration process: regulatory scientists and evaluation scientists. The text box describes these two position types’ primary duties. Neither the Federal Insecticide, Fungicide, and Rodenticide Act nor the provisions of state law governing the pesticide registration process have specific training requirements for DPR staff regarding the evaluation of registrant-submitted data.
DPR Scientists’ Primary Registration Duties
Regulatory Scientists:
- Reviewing applications, including determining the pesticide label’s compliance with California and federal label requirements.
- Routing applications through scientific evaluations, if necessary.
Evaluation Scientists:
- Performing scientific evaluation of pesticides’ efficacy and potential hazards.
- Producing the evaluation report for their respective evaluation station.
Source: DPR’s Pesticide Registration Process Desk Manual.
DPR uses a one-year, curriculum-based training program to train its new regulatory scientists on how to conduct the registration process. The training curriculum indicates that regulatory scientists learn the federal and state requirements related to pesticide use, data, and labeling, as well as in DPR-specific business processes. In 2014 DPR reported that regulatory scientists did not believe that they were receiving consistent, effective training in part because multiple trainers delivered the training. DPR addressed this feedback by appointing a senior environmental scientist as the dedicated trainer. He asserted that the training curriculum provides value in preparing the new regulatory scientists for their job duties but that DPR does not have a formal process for evaluating its effectiveness.
DPR implemented a new, more formalized version of the standardized training for regulatory scientists in August 2023. DPR indicated several goals for the new training, including improving the process of tracking staff progress; formalizing feedback between the training participants and the trainer, training committee, and supervisor; and establishing greater visibility into the training process across the registration branch. Because it believes that it is able to determine whether staff receive training and because no issues about a lack of training have come up recently, DPR has not been requiring the registration branch to track staff progress in receiving the new training. However, DPR indicated that with an increase in hiring staff in the branch, documenting progress through the training program will become reasonable and necessary going forward. We determined that, since August 2023, two out of three supervisors have been confirming the training progress new staff have made.
DPR explained that it relies on hiring requirements and on-the-job training to ensure that its evaluation scientists are able to effectively carry out their registration duties. Several positions, including specialist and supervisor roles, perform this work, depending on the complexity and expertise necessary. To progress to a more advanced role, scientists must have either significant on-the-job experience in research, analysis, or environmental monitoring or have the educational equivalent of a master’s or doctorate degree.
In contrast to its standardized training program for regulatory scientists, DPR indicated it has not established a formal standard against which to compare the training of evaluation scientists. Instead, while on the job, new evaluation scientists gain experience on the protocol and procedures related to the registration and evaluation process through close supervision and peer mentoring. According to DPR, this process includes management’s assigning applications to scientists based on the complexity of the application and the experience level of the scientist. Because of the scientific expertise required to be in the role and the absence of specific training requirements in the previously mentioned laws, we believe that DPR’s on-the-job training process for evaluation scientists is appropriate.
Changes to DPR’s Label Amendment Processes
State law requires registrants to attach a label to each pesticide product that they intend to sell in California, and that label must include the name, brand, or trademark under which the pesticide product is sold, as well as other information. DPR reviews these labels as part of its registration process. After a pesticide product is registered with DPR, a registrant can revise a product through an amendment, notification, or non-notification process, as the text box describes. Registrants can submit all types of label and formulation revisions to DPR through the amendment process. In contrast, only a limited number of revisions may be submitted through the notification process, and there are very few revisions that qualify for the non‑notification process.
Methods for Revising Labels
Amendment: Product label or formulation revisions requiring submission through the amendment process must be reviewed and accepted by DPR before the distribution or sale of the product in California. For example, changes to the use rates, the addition of use sites, crops, and pests, reducing signal words (such as DANGER) or precautionary language, or changes in the percent of an active ingredient must all be submitted as an amendment to DPR.
Notification: Certain label or formulation changes may be submitted as a notification. For example, removing a pest or use site, redesigning the label, and correcting typographical errors can all be submitted as a notification. These types of changes do not require scientific evaluation and therefore, do not require a public report or public comment period.
Non-notification: Some revisions to a product’s label or formulation are allowed without the registrant being required to submit a notification to DPR. For example, changes in the source of an inert ingredient can be submitted through the non-notification process.
Source: DPR Pesticide Registration Process Desk Manual and California Notice 2002-1.
DPR asserted that it is in the process of revising its regulations and that it plans to include a change to make the label amendment process more efficient. Specifically, DPR plans to allow applicants to complete the same form regardless of whether they are applying through an amendment or a notification method of revision. DPR has not yet filed a notice of proposed action to initiate the formal rulemaking process. Once that occurs, the Administrative Procedure Act gives DPR one year to complete the rulemaking process and submit the completed rulemaking file to the Office of Administrative Law for approval, which may take up to 30 additional days.
In 2022 and 2023, DPR similarly implemented two changes that updated its internal processes for reviewing pesticide applications, including those for amending labels. First, to shorten its response time to registrants, it changed the way it processes expedited applications for scientific evaluation, which includes label amendments. Although DPR stated that it does not receive many of these types of submissions, expedited applications are urgent and DPR prioritizes them over other submissions that may currently be under scientific evaluation. In December 2023, DPR sent a guidance email to staff indicating that it will email acceptance letters and scanned labels to registrants when label amendments are marked as expedite, a designation which the branch chief must approve. Before this update, DPR’s procedure was to respond to all amendment applications by mail.
Second, DPR revised its action log procedures to decrease processing times for all applications, including label amendments. Effective October 2022, regulatory scientists stopped submitting weekly action logs to their supervisors for review; instead, to document the actions taken, supervisors or the routing coordinator now fill in a master action log as they sign off on registrations, including amendments. These documented actions can include proposals to accept a revision with notice or proposals to deny a revision with notice. Previously, a routing coordinator processed actions on a weekly basis, and a week or more could pass between when a supervisor reviewed the submission and when the action could be taken. Now, most actions can be taken as soon as the supervisor or manager approves the application, decreasing the number of days to process an amendment. Further, using the master action log limits the number of staff entering information and should decrease errors within DPR’s tracking database.
In addition, DPR implemented an external change in 2022 to revise its procedures for handling how registrants apply for company name changes. This change reduced paperwork and processing time frames for registrants and DPR. Previously, DPR required registrants to submit a new application and affidavit for each product registered by the company for which the registrant wanted to request a company name change. DPR no longer requires registrants to submit a separate company name change application for each registered product. Additionally, applications that only require a company name change will be processed separately from product amendments, and registrants may submit their requests through email rather than by mail. An environmental program manager for the registration branch stated that this change is decreasing processing times for amendment applications.
Recommendations
To determine whether it is meeting its obligation to perform timely registration reviews, DPR should, by January 2025, establish a plan to develop, implement, and meet valid and measurable standards, including any required by law, for registration processing times. Unless AB 2113 is enacted, DPR should adopt regulations reflecting its planned timeliness standards by January 2028. To provide transparency and demonstrate progress as it works toward meeting those standards, DPR should, by January 2025, develop and implement a policy to annually report the following on its website:
- Its progress in meeting interim targets for its average registration processing times, for the number of applications it will process annually, and for reducing existing backlogs.
- Its progress in adopting or revising its regulations to reflect its updated timeliness standards.
To ensure that its implementation of CalPEST sufficiently addresses its current tracking system’s limitations related to simultaneously processed applications, consistent renewal processing, and determination of evaluation times by application use type, DPR should, by July 2025, demonstrate that it is using CalPEST to do the following:
- Track registrations that it is processing simultaneously.
- Automate the registration renewal process, including standardizing the process’ procedures and supporting documentation.
- Track registrations by use-type.
To ensure that it can perform timely reviews for pesticide registrations, DPR should, by July 2025, develop a policy to assess its registration-related staffing needs on at least an annual basis by monitoring its progress toward achieving valid and measurable overall and station-specific timeliness standards. The policy should identify how it will measure the effectiveness of its efforts to improve its registration process through adjustments to its staffing.
To ensure that it consistently selects for audit companies with heightened risks of noncompliance with mill reporting requirements, DPR should, by January 2025, formalize its guidance for annually selecting companies to audit. The guidance should identify the main criteria DPR will employ when selecting companies to audit, such as a company’s sales volume, changes to a company’s sales volume, and a company’s geographic location. The guidance should also identify the number of audits DPR should conduct annually.
To demonstrate that it adequately trains its regulatory scientists, DPR should, by January 2025, implement a policy and procedure for tracking completion of the required training for new regulatory scientists.
We conducted this performance audit in accordance with generally accepted government auditing standards and under the authority vested in the California State Auditor by Government Code section 8543 et seq. Those standards require that we plan and perform the audit to obtain sufficient, appropriate evidence to provide a reasonable basis for our findings and conclusions based on the audit objectives. We believe that the evidence obtained provides a reasonable basis for our findings and conclusions based on our audit objectives.
Respectfully submitted,
GRANT PARKS
California State Auditor
July 2, 2024
Staff:
Michelle Sanders, PMP, Audit Principal
Josh Hooper, CIA, CFE, Senior Auditor
Annie Lloyd
Kate Monahan
Richard Power, MBA, MPP
Savanna Rowe
Meredith Wang
Legal Counsel:
Natalie Moore
Appendices
Appendix A—Processing Times by Application Type
Appendix B—Scope and Methodology
Appendix A
Processing Times by Application Type
We used DPR’s Registration Tracking System data to calculate its average time frames to process different types of pesticide registration applications, the variability of these time frames, and the number of applications it received and processed. Table A provides information on the different application types submitted to DPR. As the table shows, the time frames and variability for all application types have increased during the last five years.
Appendix B
Scope and Methodology
The Audit Committee directed the California State Auditor to conduct an audit of DPR related to its pesticide registration and mill assessment processes. Table B lists the objectives that the Audit Committee approved and the methods we used to address them. Unless otherwise stated in the table or elsewhere in the report, statements and conclusions about items selected for review should not be projected to the population.
Assessment of Data Reliability
The U.S. Government Accountability Office, whose standards we are statutorily obligated to follow, requires us to assess the sufficiency and appropriateness of computer-processed information we use to support our findings, conclusions, or recommendations. In performing this audit, we relied on electronic data files that we obtained from DPR related to the registration applications and the renewals of registrations it received from 2019 through 2023, as well as the amount of time applications remained at each evaluation station during this period.
To evaluate the data, we reviewed existing information, interviewed people knowledgeable about the data, and performed electronic testing of key elements of the data. We reviewed the completeness and accuracy of the applications and evaluation stations time frames data, and we found that the data are sufficiently reliable for reporting on the timeliness and variability of registrations. However, we identified problems with the source documentation supporting the renewals data that made us question its reliability. Specifically, we found that the renewal data were complete but that DPR had inconsistent procedures and supporting documentation that affected our ability to test its accuracy. Therefore, we found the renewals data to be of undetermined reliability. Although we recognize that these limitations may affect the precision of the numbers we present, there is sufficient and appropriate evidence to support our findings, conclusions, and recommendations.
Response
Department of Pesticide Regulation
June 20, 2024
Grant Parks
California State Auditor
621 Capitol Mall, Suite 1200
Sacramento, California 95814
Subject: Response to Draft Report No. 2023-128 Department of Pesticide Regulation – Registration and Assessment Funding
Dear Mr. Parks:
Thank you for the opportunity to review and respond to the California State Auditor’s draft report 2023-128.
We appreciate your office’s thoughtful review of our programs and recognition of the important role pest management and pesticide regulation play in protecting public health and the environment. We have reviewed the recommendations in the audit report which are largely in alignment with our current efforts to continually improve our programs and are happy to share that we are already in the process of their implementation.
DPR’s mission is to protect human health and the environment by fostering sustainable pest management and regulating pesticides, with the vision of a California where pest management is safe, effective, and sustainable for everyone. Pest management plays an integral role in the production of a stable, healthy food supply, the public health of our communities, and the maintenance of our infrastructure. DPR’s work includes registering pesticides sold or used in California, including conducting scientific evaluations of pesticides, to assess and mitigate potential harm to human health and the environment.
As a part of DPR’s registration and evaluation programs, DPR receives and processes approximately 5,000 submissions each year for new products, product changes, and additional data. Collectively, DPR maintains registrations by approximately 1,500 pesticide product and device companies and for approximately 13,100 pesticide products that collectively contain over 1,000 different active ingredients.
DPR registration decisions are subject to the California Environmental Quality Act (“CEQA”). DPR is committed to meeting its legal obligation to conduct a thorough and scientifically-robust review of pesticide registrations to protect human health and the environment.
Over the last several years, DPR has made changes to improve the consistency, transparency, predictability, and efficiency of its registration program while maintaining the integrity of the required scientific evaluations. These changes, which pre-date the audit findings and recommendations, include conducting preliminary scientific consultations to confirm routing is necessary, issuing an annual public report on registration timelines, and reorganizing workloads to focus on active ingredients rather than registrants. As the CSA notes, DPR is also in the final stages of developing the California Pesticide Electronic Submission Tracking (CalPEST) system, which will shift the department from a paper-based to an electronic registration system. CalPEST will allow DPR to electronically centralize and route to staff pesticide product information, scientific studies, and reviews. CalPEST will also provide better visibility to registrants on their application status and permit secure electronic payments. Initial system launch is scheduled for Fall 2024. The updated system will introduce many process efficiencies such as simultaneous routing to and review by scientific evaluation stations wherever possible. In addition, the modernized system will provide vastly improved data collection on registration packages. The system will also facilitate reporting progress towards registration time standards and other registration program improvements.
In addition to these changes, DPR has previously noted that the program is currently understaffed to perform the necessary scientific and technical reviews of pesticide products, resulting in backlogs for registration actions. ① As a result, DPR requested and received six positions in FY 23/24 to address the most significant delays in evaluation and review of products. Governor Newsom’s 2024-2025 budget includes a proposal for Sustainable Funding for Pest Management to provide long-term, stable and sustainable funding for DPR. The funding will support streamlining processes, strengthening statewide services and providing support for local partners, communities and stakeholder. ② A component of the 2024-2025 budget proposal is DPR’s request of an additional 31.2 registration and evaluation-focused positions in FY 24/25 to further improve and streamline the programs with the goal of eliminating backlogs in evaluation stations by 2026. At the time of submission of this letter, discussions around this proposal are ongoing with the Legislature.
Collectively, the above-described actions are designed to address the existing challenges for thorough, timely, and transparent registration reviews and actions.
DPR finds that the CSA’s recommendations align with the department’s focus on improving the efficiency, consistency, and transparency of its registration program. DPR looks forward to working with the CSA on additional reporting on our achievement of these recommendations.
Sincerely,
Julie Henderson
Director, Department of Pesticide Regulation
Comments
California State Auditor’s Comments on the Response From the Department of Pesticide Regulation
To provide clarity and perspective, we are commenting on DPR’s response to our audit. The numbers below correspond to the numbers we have placed in the margin of DPR’s response.
① DPR states that it requested and received six positions in fiscal year 2023–24 to address the most significant delays in evaluation and review of products. We report that in its fiscal year 2023–24 budget change proposal, DPR requested seven additional positions to, among other things, start addressing the needs in the registration program. We report the total number of positions DPR requested, while DPR reports the number of positions requested that would specifically support the registration program.
② DPR states that as part of its fiscal year 2024–25 budget change proposal, DPR requested an additional 31.2 registration and evaluation-focused positions. We report that 36 of DPR’s requested positions would be funded by the registration fees. In this case, DPR is reporting the number of positions focused on the registration program. We report the total number of positions, including some administrative positions, that would be at least partially funded with registration fees.
Footnotes
- DPR is authorized to collect an additional three-fourths mill, or 0.075 cents, per dollar of sales of specified pesticides to support certain consultation services provided by the California Department of Food and Agriculture. ↩︎
- Pest control dealers include any manufacturer, distributor, or retailer who sells pesticides to users for agricultural use, sells to users any method or device for the control of agricultural pests, solicits sales of agricultural-use pesticides through field representatives or other agents, or sells to a user a pesticide legally classified as a restricted material. Pesticide brokers are entities that sell or distribute registered pesticides in California and that are not licensed pest control dealers or registrants selling their own products. ↩︎
- This law applies only to the initial registration of new pesticide products and does not apply to label amendments. ↩︎
- Because of limitations in validating DPR’s data for processing times related to regulatory scientists’ review, we do not include a separate analysis for the amount of time regulatory scientists spend reviewing applications. However, the overall analysis in Table 1 captures the time spent for all phases of the application review. ↩︎
- The oversight entity for IT projects in California is the CDT. Its duties include approval of SPRs for certain IT projects. An SPR is generally required when a project substantially deviates from its approved costs, benefits, or schedule. For SPR dates, we use the date that CDT approved the SPR. ↩︎
- DPR has made only one change to CalPEST’s scope, and that change has a limited effect on CalPEST’s overall functionality. In its fourth SPR, the department removed the development of one feature from the project’s scope because of the feature’s complexity, security needs surrounding that process, and the ability of current DPR staff to maintain and enhance the existing system supporting the process. DPR indicated in its SPR that the removal of this process would have limited impact on the remaining CalPEST system. ↩︎