
2023-120 Drug and Alcohol Treatment Facilities
They Are Sometimes Concentrated in Residential Areas, as Allowed, but State Oversight Is Not Always Timely or Thorough
Published: October 24, 2024Report Number: 2023-120
October 24, 2024
2023‑120
The Governor of California
President pro Tempore of the Senate
Speaker of the Assembly
State Capitol
Sacramento, California 95814
Dear Governor and Legislative Leaders:
As directed by the Joint Legislative Audit Committee, my office conducted an audit of the Department of Health Care Services (Health Care Services), and our assessment focused on residential drug and alcohol recovery and treatment facilities (treatment facilities) that provide services—such as detoxification and counseling—for substance use disorders. The following report details our audit’s determination that Health Care Services is not required to and does not limit the geographical concentration of treatment facilities. We also found that Health Care Services does not always conduct prompt compliance inspections or complaint investigations of facilities.
Some residents in Orange County have expressed concerns that an overconcentration of treatment facilities is negatively affecting their communities, and we did find that Southern California and other specific geographic areas throughout the State contain groupings of treatment facilities that serve six or fewer residents (small facilities) in residential areas. However, state law does not limit the number of treatment facilities that may operate in a given area, and the law mandates that small facilities be considered a residential use of property; therefore, local authorities may not use zoning to prohibit small facilities from operating in residential areas. We found several small facilities with the same owner located next door or across the street from each other in residential neighborhoods in Orange County and in San Diego County. If such concentrations of small facilities are not consistent with the law’s intent, the Legislature could potentially change state law.
We found that Health Care Services appropriately reviewed treatment facility license applications, but it was late in completing inspections for half of the 26 facilities that we reviewed. Further, the department took more than a year to complete 22 of the 60 complaint investigations that we reviewed, despite internal guidelines to complete such investigations within 30 to 60 days. In addition, Health Care Services has not adequately followed up with certain unlicensed facilities that it determined were providing or advertising services that require a license, such as detoxification, to ensure that the facilities were not unlawfully providing services. However, we did find that, when the department identified patterns of serious deficiencies in licensed treatment facilities, it appropriately suspended and revoked those facilities’ licenses.
Respectfully submitted,
MIKE TILDEN, CPA
Chief Deputy State Auditor
Summary
The Department of Health Care Services (Health Care Services) licenses residential drug and alcohol recovery and treatment facilities (treatment facilities) that provide substance use disorder services, such as detoxification and counseling (treatment services). To protect the health and safety of treatment facility residents, Health Care Services inspects facilities to ensure that they meet requirements in state law and investigates complaints about facilities and treatment counselors. Our audit focused primarily on these residential treatment facilities, with a specific review of the concentration of treatment facilities serving six or fewer residents (small facilities) in certain residential areas. Our audit found the following:
Health Care Services Is Not Required to Limit the Geographical Concentration of Small Treatment Facilities and Does Not Do So
Some California residents have expressed concerns that an overconcentration of treatment facilities is negatively affecting their communities. However, state law does not limit the number of treatment facilities that may operate in a given area—such as a residential neighborhood—and thus Health Care Services does not monitor or limit the concentration of these facilities. There were approximately 500 small facilities in California in 2023, and we identified groupings of such facilities in specific geographic areas throughout the State. For example, we found several small facilities with the same owner located next door to or across the street from each other in residential neighborhoods in Orange County and in San Diego County.
State law mandates that small facilities must be considered a residential use of property for purposes of any zoning ordinance. Because local authorities may use zoning requirements to regulate facilities serving more than six residents (large facilities) more strictly than small facilities, some facility operators may avoid certain zoning regulations by intentionally grouping small facilities in the same geographic area instead of establishing one large facility. The Legislature could potentially change state law if these facility concentrations are not consistent with the law’s intent, which we believe was to integrate residents of these facilities into the communities and to provide for sufficient numbers and types of treatment services to meet local needs.
Health Care Services Licenses and Certifies Treatment Facilities Appropriately, but It Has Conducted Inspections Late Since the Pandemic Began
In reviewing the department’s license application process, we found that Health Care Services consistently reviewed the 26 applications for licenses and certifications that we assessed and that it conducted the required initial on‑site inspections for each facility. Health Care Services’ process to license treatment facilities is the same for all facilities, regardless of the number of residents that the facility will house, but facilities with more than six residents must satisfy some additional requirements in state law, involving insurance coverage and zoning approvals. When we reviewed 26 compliance inspections that Health Care Services conducted as part of the license renewal process for operating facilities, we found that the department only conducted half of those inspections on time. Instead of inspecting operating facilities within the two‑year licensure period prescribed by state law, Health Care Services conducted these compliance inspections with a median delay of 207 days beyond its prescribed deadline, which is 90 days before the expiration of each license. These delays may have led to the late discovery of health and safety deficiencies that could have endangered residents. For example, during one late inspection we reviewed, Health Care Services discovered that some of a facility’s employees did not have proof of authorization to provide treatment services.
Health Care Services Does Not Always Promptly or Thoroughly Investigate Complaints
Health Care Services investigates complaints about treatment facilities; however, we found that it is not always timely in completing these investigations. Specifically, although required to assign a complaint to an investigator within 10 days, Health Care Services frequently does not assign complaints on time. We found that it took Health Care Services an average of 183 days to assign the complaints when it did not meet its 10-day required time frame.
In addition, although state law does not require that Health Care Services complete its investigations within a specific time frame, the department’s internal guidelines generally identify that investigative reports must be submitted by the analyst to a supervisor within 30 to 60 days. However, we found that it took Health Care Services analysts nearly one year on average to submit investigative reports for low‑ and medium‑priority complaints. Health Care Services completed high‑priority investigations, such as those relating to resident deaths, within an average of less than three months but still did not meet its guidelines. When Health Care Services does not complete an investigation in a timely manner, deficiencies may go unaddressed for significant periods.
We also found that Health Care Services did not always conduct site visits when investigating unlicensed facilities and did not always follow up after completing investigations of unlicensed facilities that were unlawfully advertising or providing services to ensure that they ceased doing so. In one example, after the department had substantiated an allegation that an unlicensed facility was unlawfully providing services, we found no indication that Health Care Services conducted sufficient follow‑up to verify the facility’s claim that it was not providing services, such as by performing an on‑site visit to ensure it had ceased doing so.
To address these findings, we recommend that Health Care Services implement processes to improve the timeliness of its inspections and complaint investigations, conduct additional site visits, and appropriately follow up with unlicensed facilities to ensure that they stop providing or advertising services. We also propose that if the Legislature seeks to address concerns about the overconcentration of treatment facilities in residential communities, it could potentially enact legislation to address the issue.
Agency Perspective
Health Care Services agreed with our findings and, in some cases, has already begun implementing the recommendations that we directed to it.
Introduction
Background
Problems with alcohol and substance use affect millions of Californians, and nearly one hundred thousand Californians received treatment in 2019 to address those issues. Addiction is recognized as a treatable disease by the National Institute on Drug Abuse. Although some individuals receive outpatient treatment, others may need full‑time residential care in a treatment facility to meet their needs. The State oversees alcohol and drug use recovery and treatment facilities (treatment facilities) that provide substance use disorder treatment to individuals. These services can include detoxification, counseling, and recovery or treatment planning.1 State law authorizes the Department of Health Care Services (Health Care Services) to oversee treatment facilities. Through this oversight, Health Care Services aims to promote and protect the health and safety of communities and consumers with substance use disorders.
Treatment Facility Licensing
Health Care Services is responsible for licensing residential nonmedical treatment facilities that provide certain services, listed in the text box. State law requires facilities to obtain a license before providing any of these services. When Health Care Services approves a treatment facility’s license, it also approves the specific services that the facility may provide. As a result, a treatment facility may not provide any services beyond those specified on its license. For example, only facilities whose licenses authorize them to provide detoxification or other incidental medical services may do so. State regulations require all treatment facilities to have 30 percent of their alcohol and drug counselors on staff be licensed or certified.
Services That Licensed Treatment Facilities May Provide
• Detoxification
• Individual counseling sessions
• Group counseling sessions
• Educational sessions
• Incidental medical services
• Recovery planning
Source: State law and regulations.
To receive a license, a treatment facility must file an application with Health Care Services that demonstrates that it meets all requirements in licensing statutes and regulations and will comply with Heath Care Service standards. The facility must also obtain a fire clearance from the State Fire Marshal or the local fire authority, obtain valid insurance coverage, and pay an application fee that ranges from $3,660 to $4,882—depending on the license or certification type—plus $389 per bed.2 Health Care Services next conducts an initial on‑site inspection before allowing the facility to accept residents. These licensing requirements generally apply to all facilities, regardless of the facility’s resident capacity or location. As we show in Figure 1, nearly 1,000 residential treatment facilities in the State have active licenses each year, with 500 serving six or fewer residents and the remaining 480 serving more than six residents as of June 30, 2023.
Figure 1
Nearly 1,000 Treatment Facilities in California Have Active Licenses to Provide Drug and Alcohol Treatment Services
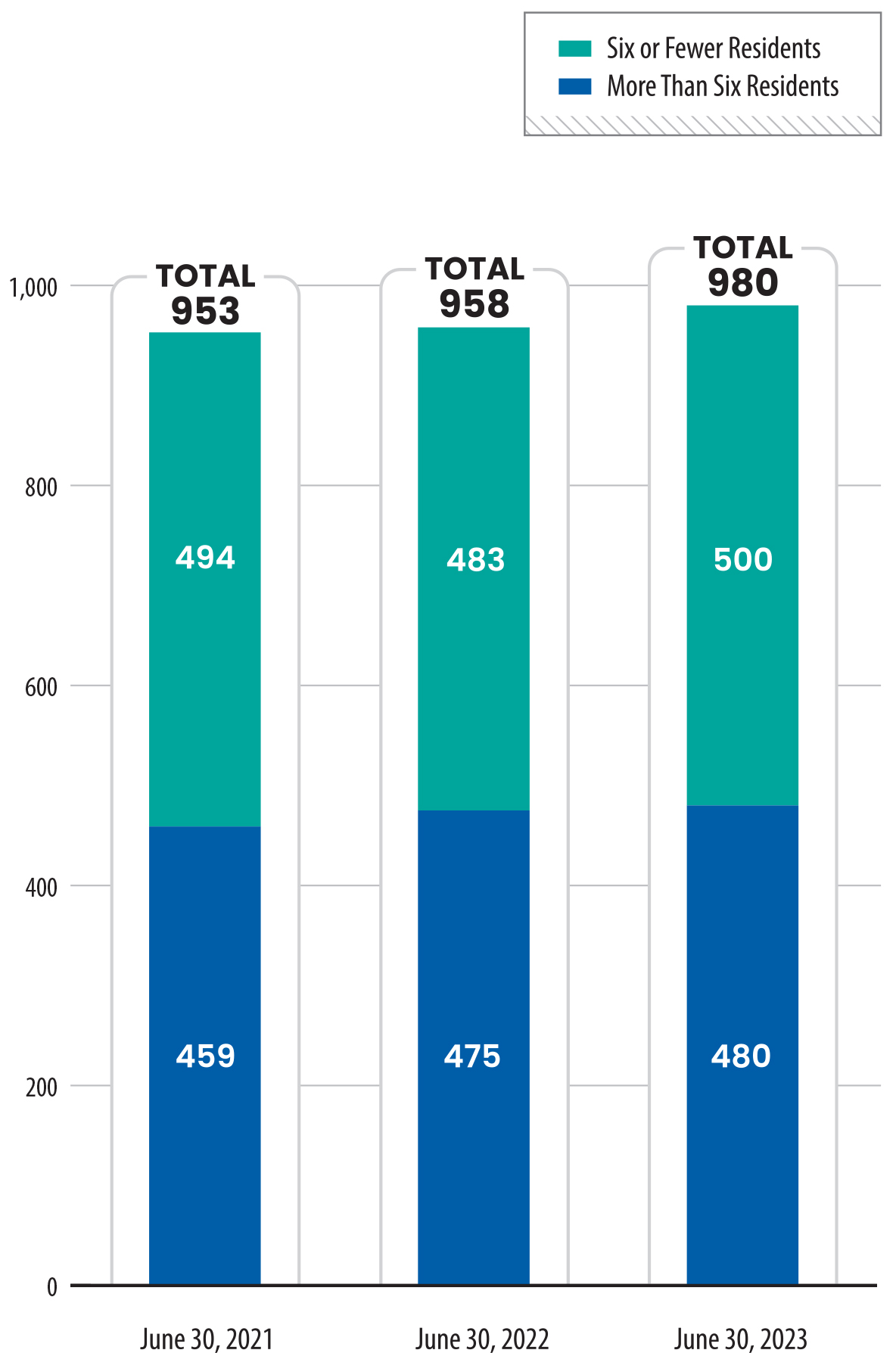
Source: Health Care Services licensure data.
Figure 1 is a bar chart showing the total number of residential treatment facilities licensed to provide drug and alcohol treatment services in California, broken down into two categories: those serving six or fewer residents, and those serving more than six residents. The X-axis represents three time points, June 30 of years 2021, 2022, and 2023, while the Y-axis shows the number of facilities, ranging from 0 to 1,000. In 2021, there were 494 small facilities and 459 larger ones. In 2022, there were 500 small facilities and 475 larger ones. In 2023, there were 500 small facilities and 480 larger ones, reflecting small but steady increases in both categories over the three years.
In addition to assessing Health Care Services’ process for licensing treatment facilities, the Joint Legislative Audit Committee directed us to assess the department’s processes for certifying treatment facilities. Health Care Services offers a voluntary certification to both residential and nonresidential treatment programs—the latter of which may only provide outpatient services. This optional certification affirms that those treatment facilities’ services exceed minimum levels of service quality and are in substantial compliance with state standards for drug and alcohol treatment certification.3 As of June 30, 2023, there were 530 residential treatment facilities, which are licensed, and nearly 860 nonresidential treatment facilities, which are not licensed, with active certifications in the State. According to Health Care Services, certification can be advantageous for treatment facilities for a variety of reasons, including increasing public awareness of the efficacy of their programs, which may attract more residents or attract greater participation by insurance companies. Additionally, according to Health Care Services, many counties require treatment facilities to be certified before they are eligible for certain types of funding.
To become certified, a licensed facility must meet some additional requirements, such as developing treatment plans for all clients that include evidence of how the treatment services are integrated and delivered, focusing on the specific needs of the individual receiving treatment. A facility must also pay an application fee to Health Care Services, file a complete application that documents the facility’s ability to meet certification standards, and pass an on‑site inspection related to the services it would be certified to provide.
Under specific circumstances, Health Care Services may terminate or deny an application for licensure or certification. If a treatment facility’s application is incomplete or requires modification, Health Care Services will notify the applicant of the issue and provide 60 calendar days for the applicant to provide the missing information or modified documentation for a license or 45 working days for a certification. Applicants also have 30 days to address any deficiencies identified during the initial on‑site inspection of the facility. If a treatment facility cannot demonstrate that it meets licensing or certification requirements after the department provides these opportunities to correct any deficiencies, Health Care Services may terminate its review of the application. If Health Care Services terminates its review, the applicant may reapply for a license at any time. In rare instances, such as when the owner of an existing facility with outstanding deficiencies or unpaid civil penalties applies for a new license, Health Care Services may deny the application. Health Care Services may also deny applications for certification for reasons specified in state law, such as a facility’s failing to resolve deficiencies identified during an initial inspection. When Health Care Services issues a denial, an applicant has the right to appeal the decision through a hearing with an administrative law judge of the Office of Administrative Hearings. Applicants have 15 days from the notice of denial to request a hearing.
Treatment Facility Inspections
Licenses and certifications issued by Health Care Services are valid for two years. After the department issues a facility license, state law requires Health Care Services to conduct another on‑site inspection (compliance inspection) of the treatment facility at least once during that two‑year period to ensure that the facility still complies with licensing or certification requirements. If a facility complies with all requirements, Health Care Services will automatically extend the facility’s license or certification for another two years. If a facility does not address deficiencies or fails to comply with all requirements, Health Care Services will suspend or revoke the license or certification. In addition to conducting these inspections, Health Care Services also inspects facilities when they make changes to their operations, such as by increasing resident capacity or moving to a new location. Health Care Services may also inspect a facility as part of a complaint investigation.
Treatment Facility Complaints
State law requires Health Care Services to investigate complaints about treatment facilities and about treatment facility counselors who are licensed by the State or certified by other organizations to provide counseling services. Complaints may arise from various sources that include facility residents, neighbors, staff members, or government agencies. Health Care Services must also investigate reports of resident deaths. According to its internal guidelines, Health Care Services prioritizes death investigations over investigations into all other types of complaints. In fact, Health Care Services aims to assign death investigations to a staff member on the day it receives the report of a death. In the course of a death investigation, Health Care Services directs its staff to perform a complete review of the facility where the death occurred to determine whether the resident’s death was related to deficiencies in the facility’s operation.
Health Care Services also receives and investigates complaints about facilities that operate without a license. If Health Care Services’ investigation finds that an unlicensed facility is providing or advertising services that require a license, the department notifies the facility that it has been violating the law. If Health Care Services obtains sufficient evidence that the facility has not stopped providing the services in question, Health Care Services may bring a civil action against the facility.
During a complaint investigation or compliance inspection, if Health Care Services finds that a facility poses serious risks to the health and safety of residents, the department may initiate a license suspension or revocation. State law authorizes Health Care Services to immediately suspend a license when these concerns are present. A suspension stays in effect until Health Care Services makes a final determination, which may include revocation, following a hearing and a proposed decision by an Administrative Law Judge.
Treatment Facility Location
State law allows treatment facilities serving six or fewer residents to operate anywhere that a single family home would also be permitted to exist. Local governments may not impose on these facilities any additional zoning requirements or fees that they would not impose on a single‑family home. Accordingly, state law prohibits local governments from imposing distance requirements on small facilities that they can impose on commercial establishments. In addition, state law regarding the licensing of treatment facilities does not prevent facilities with individual licenses from operating near one another, including when those facilities share the same owner. However, the facilities may not share residents or treatment services among separately licensed facilities. State law does not limit the number of residential treatment licenses—regardless of facility capacity—that a single owner may possess.
Issues
Health Care Services Is Not Required to Limit the Geographical Concentration of Small Treatment Facilities and Does Not Do So
Health Care Services Licenses and Certifies Treatment Facilities Appropriately, but It Has Conducted Inspections Late Since the Pandemic Began
Health Care Services Does Not Always Promptly or Thoroughly Investigate Complaints
Health Care Services Is Not Required to Limit the Geographical Concentration of Small Treatment Facilities and Does Not Do So
Key Points
- State law does not prohibit treatment facilities from being located near other treatment facilities or sharing the same legal owners. We identified numerous residential treatment facilities serving six or fewer residents concentrated in certain residential areas, particularly in Southern California.
- State law mandates that small facilities must be considered a residential use of property for purposes of any zoning ordinance. Because local authorities may use zoning requirements to more strictly regulate large facilities, some facility operators may avoid certain zoning regulations by intentionally grouping small facilities in the same geographic area instead of establishing one large facility. Some people living in certain residential areas have expressed quality‑of‑life and safety concerns resulting from the presence of small facilities in their neighborhoods.
- To determine whether treatment facilities are operating within the scope of their licenses, Health Care Services conducts compliance inspections. These inspections include a review of whether facilities are currently serving only their permitted numbers of residents and whether facilities are sharing residents with any nearby facilities. We selected three of the groupings of facilities located near one another and found that Health Care Services did not discover in those facilities’ recent compliance inspections any instances of resident‑sharing among the facilities.
Some Treatment Facilities Are Located Near One Another, Which State Law Allows
We found that Southern California contains a greater concentration of small facilities than exists in other parts of the State. As Figure 2 shows, counties in Southern California have a higher proportion of small facility beds per 10,000 residents, compared to counties in other regions of the State. Appendix A presents other statewide and local maps showing the geographic distribution of treatment facilities. In particular, we see a high proportion of beds in Orange County. The concentration of treatment facilities also varies within counties. For example, as we show in Figure A.2 and Figure A.3 of the Appendix, certain parts of Orange County, such as coastal cities in southern and northern Orange County, show higher concentrations of facilities than do inland communities in the county. Many of these facilities are located near one another, as we describe here.
Figure 2
There Are Currently More Beds for Small Treatment Facilities Per 10,000 Residents in Orange County Than in Other Counties in California
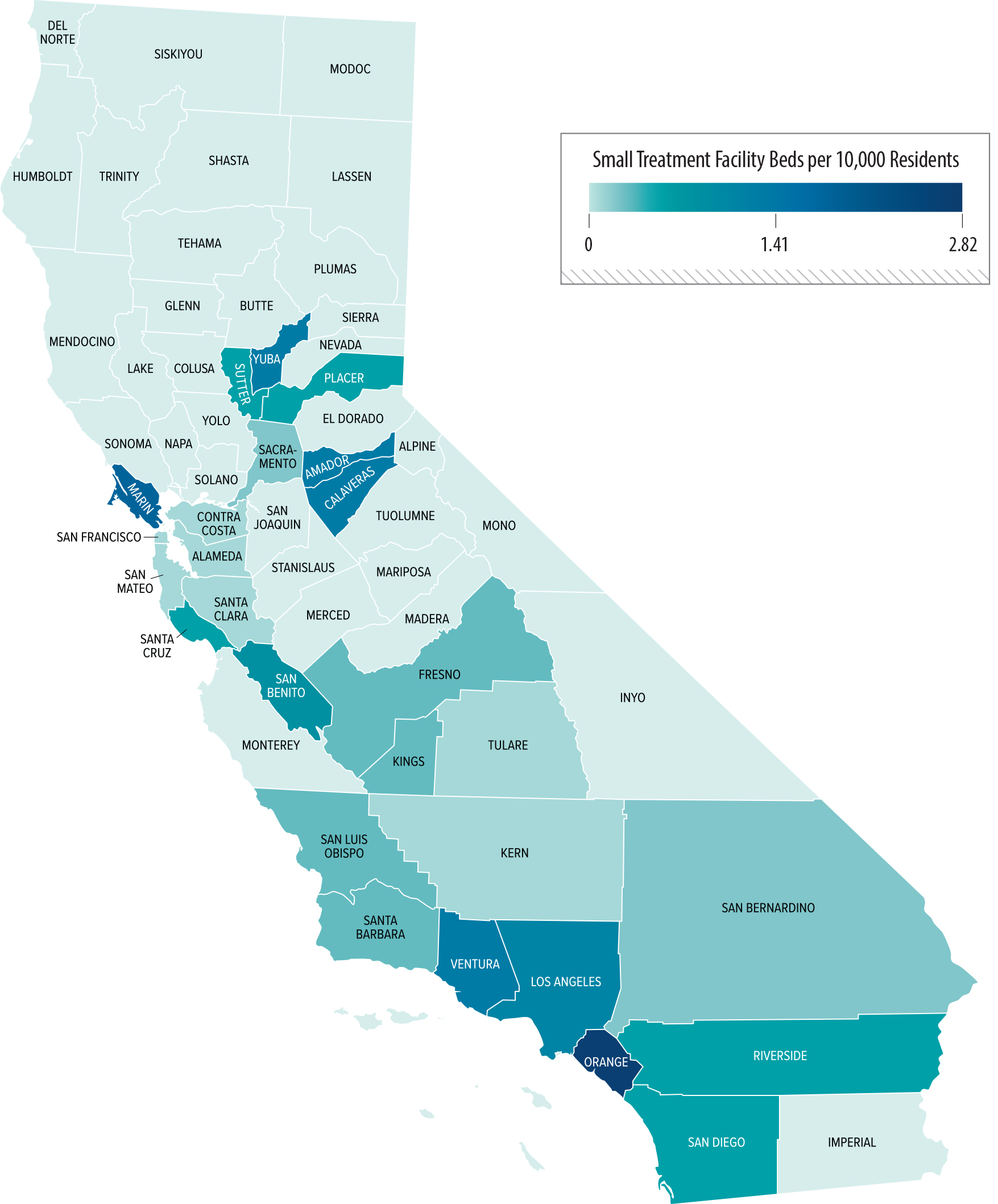
Source: Health Care Services treatment facility map of substance use disorder treatment facilities, as of July 2024, and U.S. Census 2023 population estimate data.
Figure 2 shows a map with the distribution of small residential treatment facility beds (those serving six or fewer residents) per 10,000 residents across California counties. Orange County has the highest concentration of small facility beds, with other counties, particularly in Southern California, also showing higher concentrations. The map uses shading to represent the number of beds, with darker shades indicating higher proportions of facility beds per 10,000 residents. Other regions of California, especially northern counties, have fewer beds.
Although we determined that some small facilities located in close proximity to one another share a common owner, we found that the owners have separate licenses for each facility. Within these geographic areas that have a high density of facilities, we found groupings of small facilities that were located within a short walking distance or within a few blocks of one another. We selected 10 groupings of facilities, ranging from as few as three facilities to as many as eight, and we searched ownership records to discover whether any of the facilities in these groupings had the same legal owner. Six of the 10 groupings we examined contained at least two facilities with the same owner, as Table 1 shows. We found that of the 44 facilities within the groupings, 24 were located near at least one other facility with the same owner. Three groupings were composed exclusively of facilities linked to a single owner. For example, Figure 3 shows two of these three facility groupings we identified, in which several facilities with the same owners are located within walking distance of one another. We did not identify any similar ownership ties among facilities in the remaining four groupings. State law does not prohibit owners from obtaining multiple licenses, and we found that for each of these groupings of facilities that have the same ownership, Health Care Services posted that information online, and each facility is separately licensed.
Figure 3
We Found Examples of Small Treatment Facility Groupings With the Same Legal Owner in Certain Counties
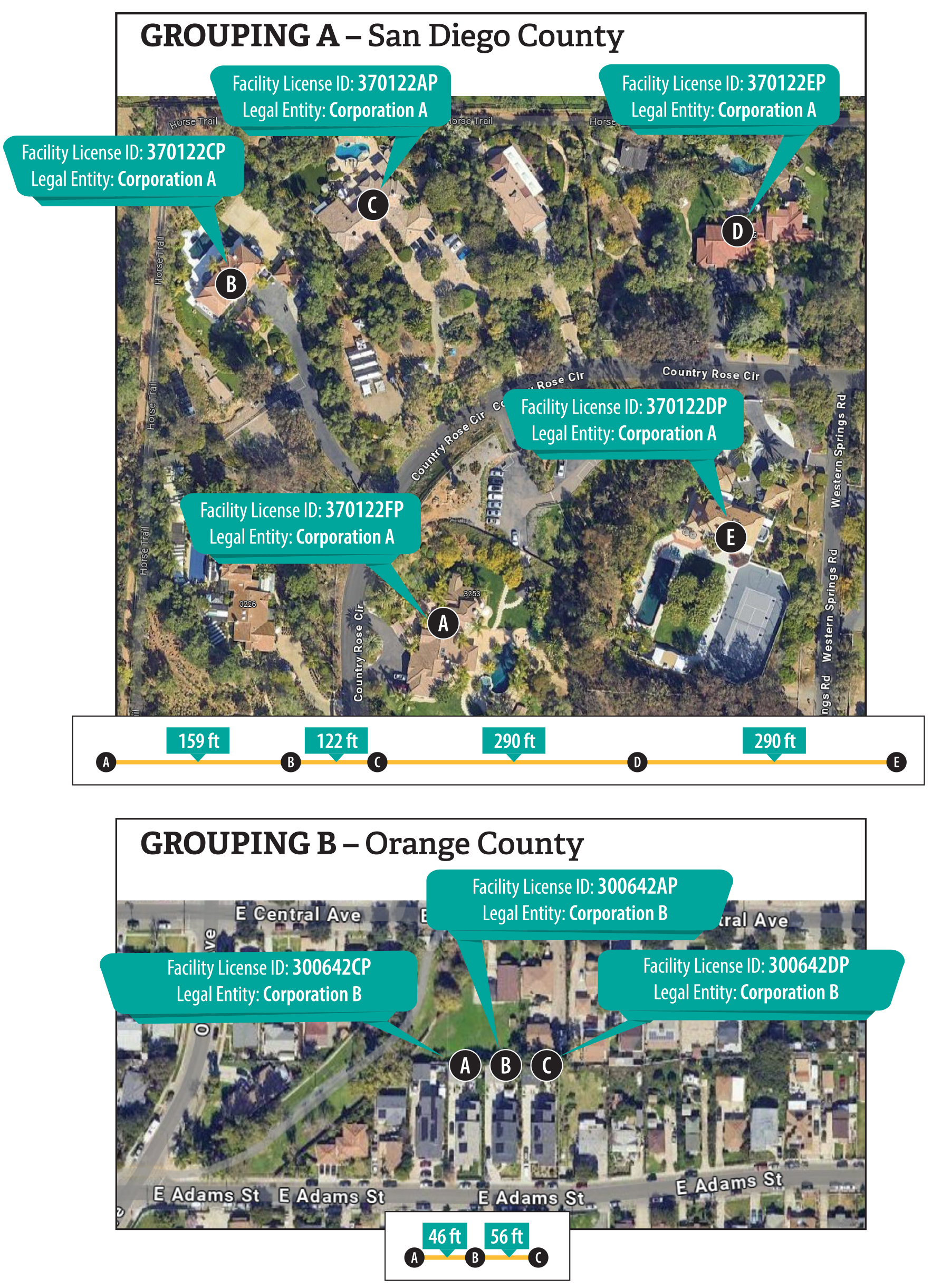
Source: Health Care Services treatment facility map, information in a Health Care Services database, and satellite images from Google Maps.
This figure consists of two maps, illustrating the geographic grouping of small residential treatment facilities with shared ownership in two counties, San Diego County and Orange County. In San Diego County, several small facilities with the same owner are located within 122 to 290 feet of one another, while in Orange County, a similar grouping of facilities is located within 46 to 56 feet of each other.
A single owner might group small facilities in a residential area in part because state law exempts such facilities from certain zoning requirements. In particular, state law establishes that treatment facilities that serve six or fewer residents will not be subject to any zoning restrictions other than those required for single family residences, and state law prohibits any fees other than those imposed on single‑family dwellings. Large residential treatment facilities may need to pay various additional fees, such as those associated with conditional use permits. These fees, which can be substantial, vary by jurisdiction and according to multiple factors, such as property size, valuation, and specific city zoning rules. These fees would not be required of small facilities because state law mandates that treatment facilities serving six or fewer persons must be considered a residential use of property for purposes of any zoning ordinance. The threshold of six residents for determining the applicability of zoning requirements reflects the average size of group homes around the time the Legislature enacted the provision. This is the same threshold used to exempt from residential zoning restrictions other types of facilities serving other populations. These include family care homes, foster homes, and group homes that serve individuals with mental health disorders or other disabilities. We believe the Legislature established these provisions for treatment facilities serving six or fewer persons to integrate residents of these facilities into the communities and to provide for sufficient numbers and types of treatment facilities to meet local needs.
Unlike the licensing statutes for facilities that serve certain other groups, state law governing treatment facility licensing does not provide any distance requirements for treatment facilities of any size. As a result, Health Care Services is not required to monitor the concentration of facilities when approving applications for licensure, and we found no indication that it does so. State law requires certain new facilities, such as those that serve persons with developmental disabilities, for example, to be 300 feet or more away from similar facilities. In these instances, the Legislature cited concern that an overconcentration of care facilities impairs the integrity of a residential neighborhood. Although this requirement is not applicable to residential treatment facilities, we did find in our review that certain small facilities were located within 300 feet of one another, as Figure 3 shows.
In response to concerns about the possible effects of an overconcentration of treatment facilities, the Legislature has previously considered several bills that would have established distance requirements for such facilities. For example, Senate Bill 786 (2017) would have required Health Care Services to deny an application for a new treatment facility license if the proposed location created an overconcentration in a residential area. The bill defined overconcentration as two or more treatment facilities separated by a distance of 300 feet or less. An analysis of the bill for the Senate Committee on Health noted concerns that the distance requirement would violate the Fair Housing Act Amendments if the distance requirement did not benefit persons recovering from alcohol and drug use and if the requirement was enacted with discriminatory intent.
Individuals recovering from drug and alcohol addiction are considered a protected class under federal disability law, as long as they are not currently using a controlled substance, and they cannot be discriminated against, such as by banning all treatment facilities from certain communities. In fact, most courts that have addressed the issue have agreed that density restrictions on group homes for the disabled are generally inconsistent with the Fair Housing Act. However, some courts have upheld local and state minimum distance requirements when they are necessary to achieve a legitimate government purpose. Moreover, the U.S. Department of Justice and the U.S. Department of Housing and Urban Development recently published a joint statement opining that it is appropriate for a government to consider overconcentration in the licensing and regulatory process. Therefore, the California Legislature could potentially enact carefully crafted legislation that addresses some concerns about overconcentration, such as possible negative effects on facility residents, without being discriminatory and without violating federal law.
Owners May Permissibly Group Small Facilities in Residential Areas and Avoid Local Zoning Restrictions
Some residents in Orange County have expressed concerns about the small facilities in their neighborhoods. During the course of our audit, we received several complaints from an advocacy organization about the concentration of facilities in a city in Orange County, including complaints about the proximity of licensed facilities to recovery residences. Recovery residences are cooperative living arrangements that support personal recovery from a substance use disorder and that do not provide licensable services and are not licensed by Health Care Services. We also reviewed complaints from certain neighborhood residents about facilities in their communities. These complaints generally related to quality‑of‑life issues, such as increased traffic, noise, or concerns about the diminishing residential character of their neighborhoods. A few complaints focused on public safety and the danger these facilities potentially pose to their communities. Many of these complaints refer specifically to the presence of sober living homes, which are recovery residences and are not licensed or regulated by Health Care Services. It is possible that some who provided this feedback are unaware of the differences between licensed treatment facilities and these residences. News media have reported on concerns and public safety incidents associated with the presence of treatment facilities in residential neighborhoods. Reports include a 2021 instance in which a resident left a licensed residential treatment facility in Newport Beach, broke into a neighbor’s home, and was killed by the homeowner.
We reviewed 10 groupings of small facilities that may speak to the concerns about the character of some residential neighborhoods. We found that in Grouping A shown in Figure 3, the five small facilities we show all share the same legal owner but have separate licenses. We also found that these five facilities advertise as a campus of connected homes, in which each facility serves six residents or fewer, as Figure 4 shows. Health Care Services asserted that these small facilities are operating within the bounds of its licensure because they do not share residents or recovery, treatment, or detoxification services (treatment services), which Health Care Services has verified. The Health Care Services compliance analyst who performed the most recent on‑site reviews of these facilities stated that although the facilities share some common amenities, such as a dining hall and a gym, the residents do not have access to other parts of the facilities in which they do not reside. The analyst stated that the residents only receive treatment services within the facility to which they are admitted. However, the affiliation of five closely located facilities that could serve a total of 30 residents may have the cumulative effect of a larger facility in a residential neighborhood.
Figure 4
One Website Advertised a Group of Small Treatment Facilities as a Campus of Connected Homes
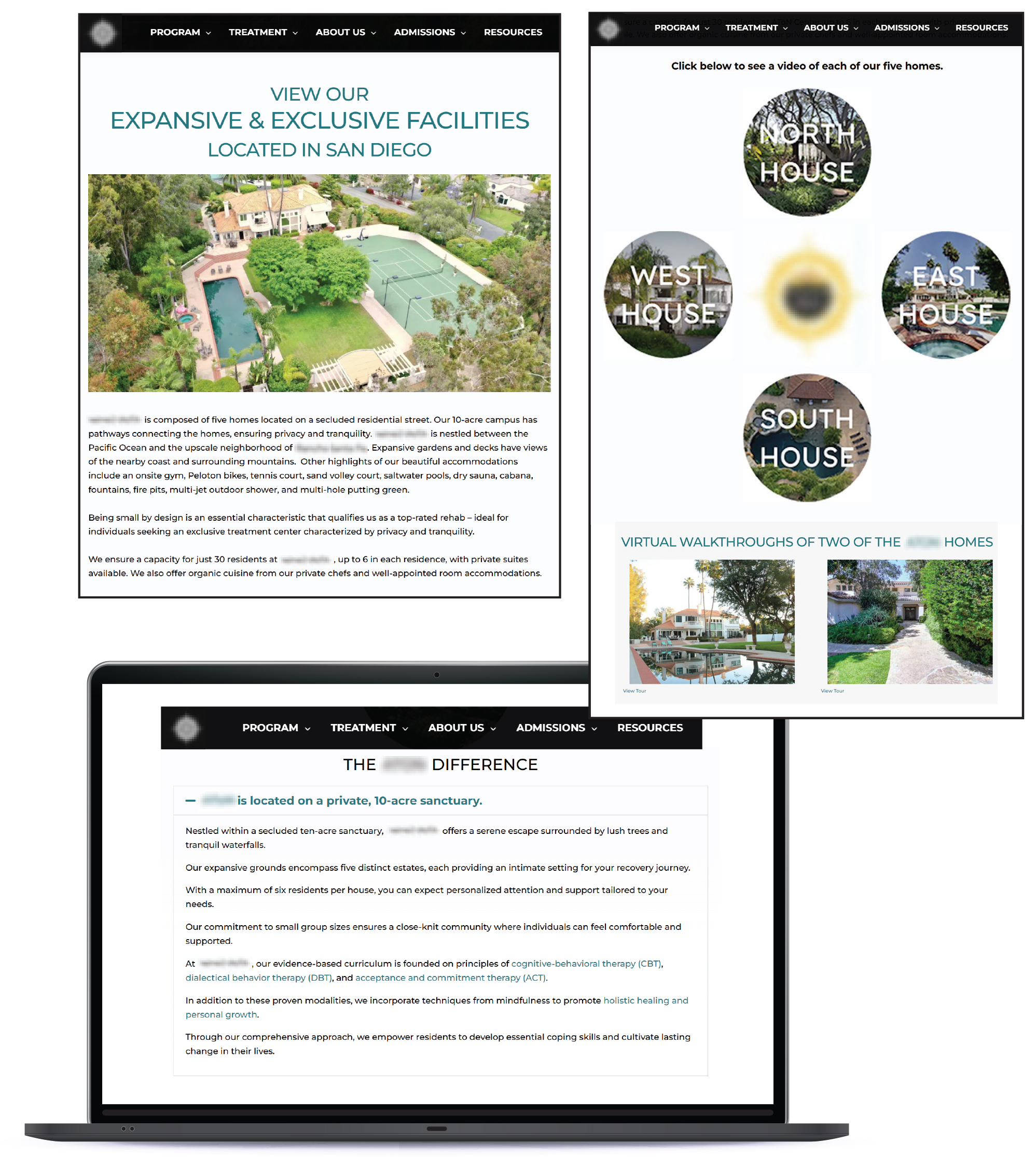
Source: Website for group of small treatment facilities, accessed in July 2024.
Figure 4 shows three screenshots of website advertisements presenting a group of small residential treatment facilities as a campus of connected homes. The image highlights how the facilities, which each serve six or fewer residents, are marketed together as part of a coordinated care system despite being separately licensed.
City and county authorities can use zoning ordinances to place restrictions on large facilities. In fact, some cities and counties have enacted restrictions or prohibitions on treatment facilities that serve more than six residents (large facilities). For example, the city of Costa Mesa does not permit large facilities to be located less than 650 feet from another facility unless the reviewing authority determines that the facility’s placement will not result in overconcentration. Orange County does not permit large facilities in multifamily residential zones in unincorporated areas of the county to be located less than 1,000 feet from another facility unless the reviewing authority determines that an overconcentration will not result. Although we did not identify any ordinances in Orange County or its cities that expressly ban large treatment facilities from operating within their jurisdictions, some of the limitations that cities or counties impose may make it impractical to operate a large facility in those communities and may encourage owners to operate multiple small facilities.
Health Care Services’ Inspections Help to Ensure That Each Treatment Facility Serves No More Than the Permitted Number of Residents
Through its compliance inspections, which it conducts every two years, Health Care Services takes steps to review whether treatment facilities—regardless of whether they have the same owner—share residents or services with any other facilities. Health Care Services conducts these inspections to ensure that treatment facilities operate within the legal limits of their licenses, including by serving no more than the permitted number of residents. State law does not allow treatment facilities to share residents or treatment services between separately licensed facilities, regardless of owner; Health Care Services stated that such sharing would violate a resident’s right to confidentiality. In particular, state law specifies that treatment facilities must provide all licensed services within the licensed facility and that only current residents may receive licensed services.4 If Health Care Services did not properly monitor facilities, the facilities might share residents and potentially serve more residents than are approved on their licenses, thereby acting as a larger facility. In so doing, they could avoid paying for the additional insurance required for large facilities and avoid local zoning restrictions.
State law permits staff to conduct on‑site compliance inspections with or without advance notice, and Health Care Services explained that it typically conducts these inspections unannounced. Because facilities do not generally know the date of an inspection, they are unable to preemptively conceal violations, such as sharing residents with other facilities. Each facility has an approved treatment capacity, and during the compliance inspection, Health Care Services analysts compare this number against the facility census and the resident files obtained on‑site. According to the department’s licensing and certification manual, analysts verify during the facility inspection that no unregistered persons are present or receiving services in the facility. Analysts must also review medication logs to confirm that only current residents are receiving medications and treatment services. After conducting physical observations of the facility, analysts are directed by Health Care Services policy to conduct interviews with residents and staff. The policy includes direction on asking questions about who receives services and where services are offered. If Health Care Services identifies compliance deficiencies, facilities can face civil penalties and possible suspension of their licenses if they do not take appropriate corrective actions.
To determine whether facilities affiliated with the same owners were violating laws that prohibit sharing residents, we selected groupings 3, 6, and 8 from Table 1 for further analysis. Having multiple facilities located in close proximity could provide an operator with an incentive to share residents or services, since economies of scale can reduce operating costs for the businesses. Accordingly, we reviewed available documentation from Health Care Services for the facilities in these groupings to identify their most recent compliance inspection, including reports of any license violations that could include incidents of sharing residents between facilities. We found that in all three groupings, Health Care Services had not identified any examples of resident‑ or service‑sharing during its on‑site inspections conducted between 2021 and 2023. We also reviewed Health Care Services’ complaint records for these facilities and did not identify any substantiated allegations of sharing. Therefore, it appears that Health Care Services’ compliance inspections can be effective in ensuring that small facilities are not sharing residents or services.
Moreover, Health Care Services uses two other structural mechanisms to support the effectiveness of its oversight of treatment facilities. The first mechanism involves caseload assignments. The department confirmed that it assigns analysts to specific geographic regions and attempts to keep facilities owned by the same person or entity with the same analyst. This allows analysts to become more familiar with the facilities in their region and the license history of those facilities, enabling more efficient inspections. Health Care Services noted that, on average, each analyst has a caseload of about 70 facilities. In the second structural mechanism, Health Care Services assigns facility licenses using alphanumeric patterns that allow analysts to quickly identify whether facilities have the same owner. These two structural mechanisms assist Health Care Services’ analysts in maintaining oversight of ownership ties among the facilities in their regions.
Health Care Services Licenses and Certifies Treatment Facilities Appropriately, but It Has Conducted Inspections Late Since the Pandemic Began
Key Points
- Health Care Services generally adhered to key requirements in state law and in its policies, such as verifying fire clearance approval and payment of fees, when approving the 26 treatment facility applications for licenses or certifications from fiscal years 2020–21 through 2022–23 that we reviewed.
- Although Health Care Services’ biennial compliance inspections of residential treatment facilities are thorough, the department did not conduct those inspections in a timely manner for half of the treatment facilities that we reviewed.
Health Care Services Generally Adhered to Key Requirements in State Law and in Its Policies When Approving Facility License and Certification Applications
Our review determined that Health Care Services generally followed licensing and certification processes and requirements when approving treatment facility applications. We reviewed a selection of 26 license or certification applications that Health Care Services approved between fiscal years 2020–21 and 2022–23, including 13 applications for small facilities and 13 for large facilities.5 As we discuss in the Introduction, state law requires treatment facility applicants to file a complete written application with Health Care Services and pay a licensure or certification fee. A complete application includes documentation of a fire clearance approved by the State Fire Marshal or local fire enforcement department; disclosures of the applicants’ ownership of, control of, or financial interest in any recovery residence; and disclosures of any other licenses or certifications issued by Health Care Services. To ensure that treatment facilities submitted complete applications, we reviewed the applications’ proposed treatment capacity, the approved fire clearance, the disclosures to Health Care Services, and the licensure fees. As Figure 5 shows, we found that Health Care Services consistently reviewed key requirements when approving the 26 applications that we reviewed.
Figure 5
Health Care Services Consistently Reviewed Key Requirements When Approving the 26 License and Certification Applications That We Reviewed
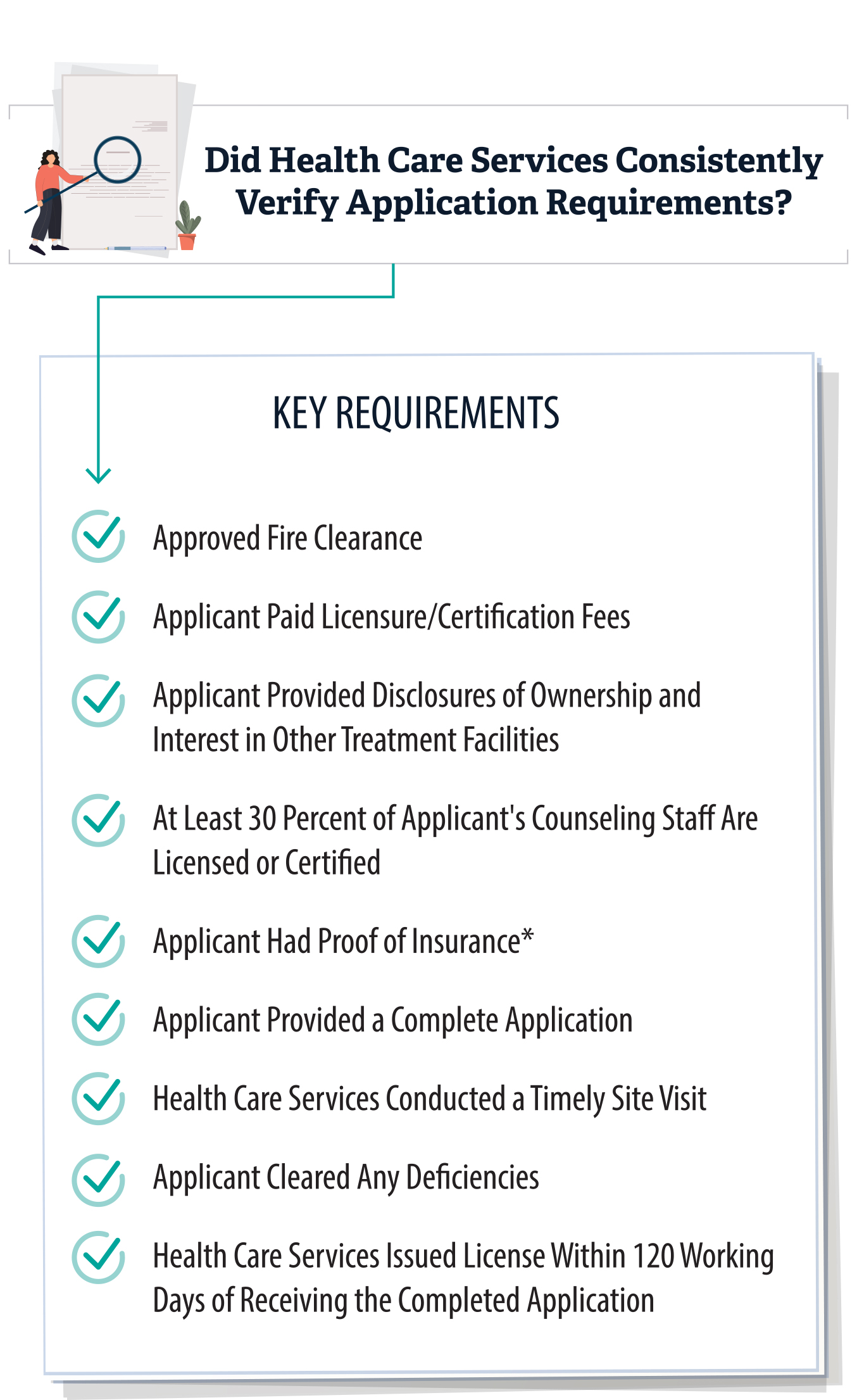
Source: Auditor analysis of Health Care Services application files from fiscal years 2020–21 through 2022–23.
* Although Health Care Services stated that it reviews the facilities’ proof of insurance during its site visits, it did not retain a copy of the applicants’ proof of insurance in its files before 2022. However, in July 2022, Health Care Services began maintaining copies of facilities’ proof of insurance, and all applications that we reviewed after that date included it.
This table outlines key requirements reviewed by Health Care Services when approving license and certification applications for treatment facilities. It lists criteria such as approved fire clearances, payment of licensure fees, ownership disclosures, staffing requirements (including the percentage of licensed or certified counselors), proof of insurance, and timeliness of site visits. The table notes that Health Care Services consistently reviewed these elements in the 26 applications reviewed.
Additionally, we reviewed the timeliness of Health Care Services’ initial on‑site inspections and approvals of applications and found that both actions occurred within required deadlines. According to state regulations, Health Care Services has 120 working days after determining that an application is complete to issue the license or notify the applicant of a denial, as Figure 6 shows. Within this time frame, state law requires Health Care Services to conduct a site visit of the treatment facility to determine the applicant’s ability to comply with all requirements for such facilities. During a site visit for an initial application, Health Care Services’ staff perform a detailed compliance review and document their actions by checking certain items, including those listed in the text box. We found that Health Care Services completed site visits within required time frames and issued licenses before the 120‑working‑day deadline for each of the 26 applications that we reviewed. In fact, for the applications we reviewed, we found that Health Care Services worked far more rapidly than regulations require, taking an average of 33 working days to conduct a site visit and issue the license after it determined the application was complete. However, we did not review the process for determining whether an application is complete, including the timeline for that process. We did not do so because application completion relies on the applicant to submit all required information and documentation; thus, the timeliness of determining that an application is complete is largely outside of Health Care Services’ control.
Examples of Initial License and Certification Application Site Visit Evaluation Items
• Facility inspection
• Verified posting of code of conduct and client rights
• Verify medication storage
• Review operations manual and policies and procedures
• Review personnel files
• Review mock client files
• Interview staff
Source: Health Care Services initial evaluation field checklists.
Figure 6
Health Care Services Has a Process to Determine Application Completeness and Issue or Deny Licenses
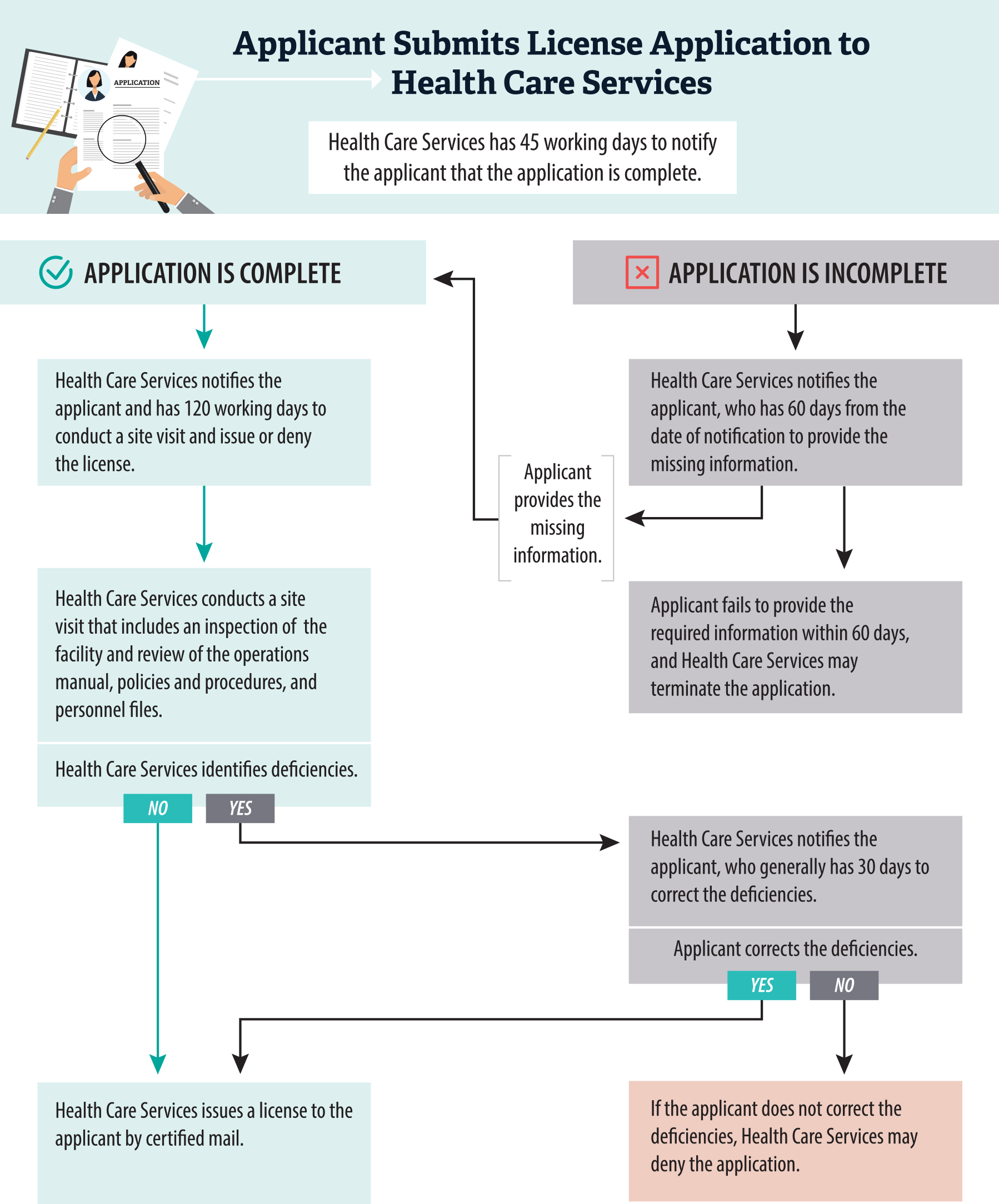
Source: State law, regulations, and Health Care Services licensing and certification manual.
This flowchart explains the step-by-step process Health Care Services uses to assess and approve or deny applications for treatment facility licenses. It begins with an applicant submitting an application, followed by Health Care Services reviewing it for completeness within 45 working days. If additional information is needed, the applicant has 60 days to provide it. If the application is complete, Health Care Services conducts a site visit within 120 working days. The chart highlights actions such as site inspections and the final decision to issue or deny the license based on compliance with all requirements.
Further, we found that when Health Care Services identified deficiencies during its initial on‑site inspection of a treatment facility, the department appropriately ensured that the applicant cleared the deficiencies. For example, in one of the applications we reviewed, an employee at a facility did not have current tuberculosis test results or a physician’s health screening report, even though state regulations require that all treatment facility personnel be in good health, verified by a health screening that includes a current test for tuberculosis. Additionally, an employee at the same facility was not certified in first aid or in cardiopulmonary resuscitation (CPR). This was problematic because the facility proposed to provide detoxification services, for which state regulations require the presence of at least one person on the premises at all times who is capable of providing CPR and first aid services. Health Care Services cited these deficiencies during its site visit and notified the program director in writing, who then corrected the deficiencies. After Health Care Services verified that the applicant had corrected the identified deficiencies, the department approved the application. In our review of 26 approved applications, we found that Health Care Services identified deficiencies during 14 of its initial on‑site inspections, including those we mentioned above, and that it licensed each of these facilities within the required 120 working days after the facility resolved the deficiencies.
Health Care Services also appropriately terminated or denied applications for licensure according to state regulations. The most common reason for termination or denial was that treatment facilities did not provide adequate documentation. The text box shows the reasons Health Care Services may terminate applications, according to state regulations. Additionally, state regulations allow the department to deny an application if the applicant is not in compliance with relevant statutes and regulations, if the applicant fails to remedy deficiencies that the department identifies, or if the applicant fails to pay any civil penalties that Health Care Services has issued. If Health Care Services denies an application for licensure instead of terminating its review, the applicant is entitled to a hearing to review the decision, as we discussed in the Introduction. We reviewed 30 applications that Health Care Services terminated or denied between fiscal years 2020–21 and 2022–23. In our review of these 30 applications, we found that Health Care Services terminated or denied 24 applications because of incomplete documentation, four because of unpaid civil penalties, and two for other reasons.
Reasons That Health Care Services May Terminate License Applications
• Department previously revoked or suspended the applicant’s license or denied an application for licensure within the previous two years.
• Applicant fails to provide required additional information within 60 days of notice.
• Applicant’s facility is denied a fire clearance.
• Applicant fails to submit the required fee for licensure.
• Applicant submits a written request to withdraw the application.
Source: State regulations.
The process for licensing and certifying treatment facilities is generally the same for small facilities and large facilities. State law governing licensure and certification does not direct Health Care Services to distinguish between small treatment facilities and large facilities during licensing or certification. However, state law does require large facilities to satisfy certain key requirements from which small facilities are exempt. Specifically, state law requires large facilities to obtain the insurance coverage listed in the text box. In comparison, the licensing statute requires that small facilities obtain only general liability insurance. Another key difference relates to the location of treatment facilities. State law requires local governments to treat small facilities as single‑family homes, which exempts them from certain zoning requirements, such as commercial zoning, which could apply to large facilities. The zoning approval process can be extensive and involve local community member feedback, and the process does not guarantee that the city or county will approve the treatment facility. Thus, being licensed as a small facility confers advantages in both insurance and zoning requirements. We reviewed Health Care Services’ approval of 26 initial license or certification applications and found that the approval process did not differ significantly by facility size.
Types of Insurance That Large Treatment Facilities Must Obtain
• Commercial general liability
• Automobile
• Workers’ compensation
• Employer’s liability
• Professional liability
Source: State law.
Health Care Services Has Conducted Mandatory On‑Site Inspections Late Since the Pandemic Began
After granting licensure, Health Care Services conducts periodic compliance inspections of treatment facilities that help to ensure the health and safety of residents. According to state law, the department must conduct on‑site program visits at least once during a facility’s licensure period, which is generally two years, to review the facility’s compliance with applicable statutes and regulations. To ensure that facilities comply with regulations, Health Care Services inspects all treatment facilities both before they can start admitting residents and once during their licensure period, as Figure 7 shows. Every two years, facilities that seek to extend their license must seek that extension from Health Care Services. To ensure that treatment facilities seeking to extend—or renew—their licenses have remained in compliance, Health Care Services’ policy states that staff will conduct an on‑site compliance inspection no later than 90 days prior to the expiration of a facility’s license.
Figure 7
Health Care Services Conducts Both Initial Inspections and Biennial Compliance Inspections
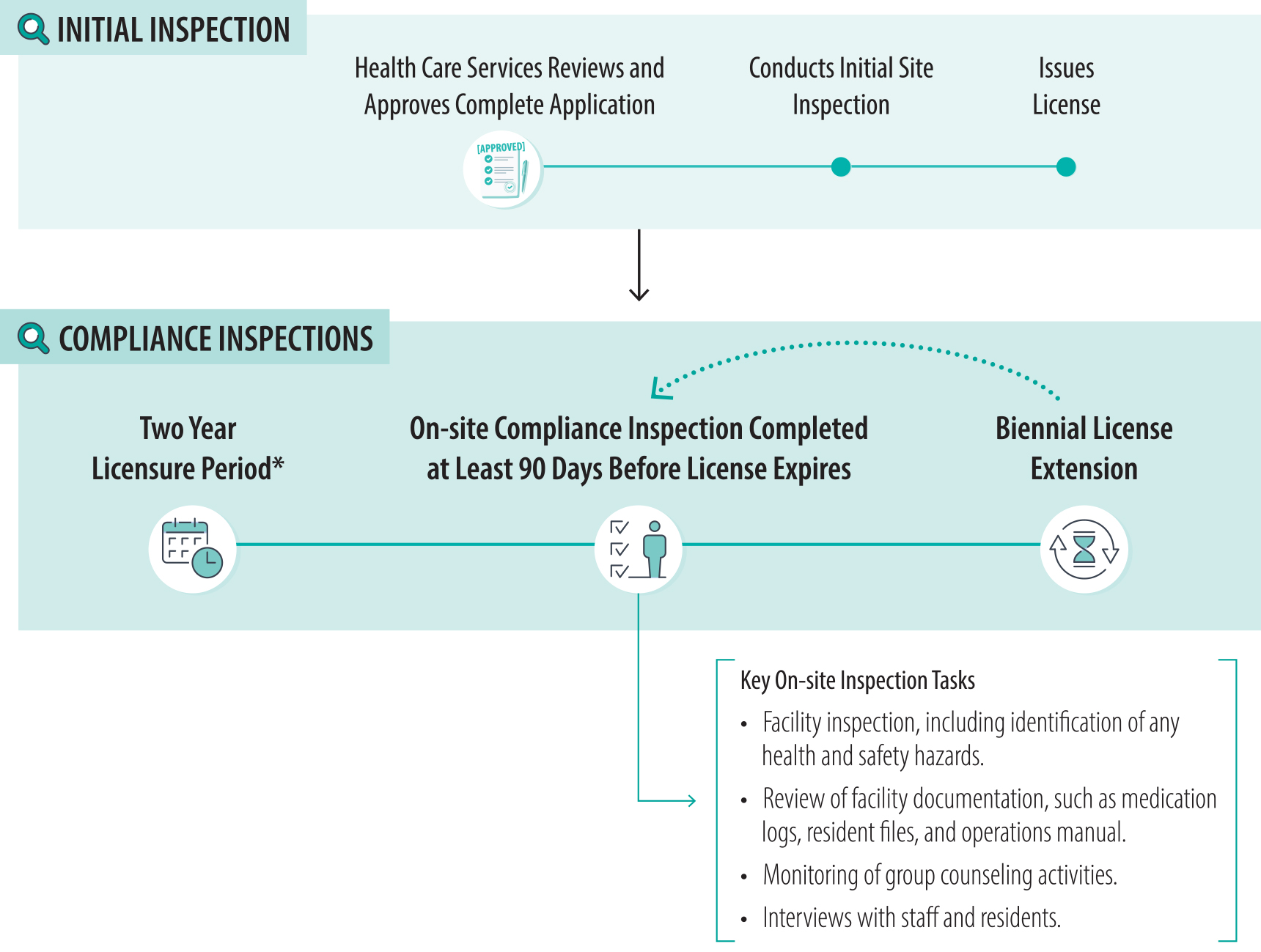
Source: Health Care Services licensing and certification manual.
* Health Care Services grants a facility an initial license, which is valid for a period of two years. The license is provisional for the first year, after which the department will allow the facility to continue to operate if it remains in substantial compliance. After this first two‑year period, facilities receive compliance inspections every two years, and their licenses are extended for a two‑year period in the cycle shown above.
This timeline explains the inspection process for residential treatment facilities licensed by Health Care Services. It includes initial on-site inspections conducted before a facility is licensed and biennial compliance inspections that take place every two years during the licensure period. The timeline emphasizes key tasks performed during inspections, such as facility inspections, reviews of operations manuals and resident files, monitoring of group counseling activities, and interviews with staff and residents.
To determine compliance, Health Care Service analysts must perform on‑site reviews and identify any deficiencies that facilities must correct. Before conducting an on‑site compliance inspection, a Health Care Services analyst must review the inspection history of the treatment facility, including past reports, complaints, and any actions Health Care Services has taken against the facility, such as a past issuance of a civil penalty for violating license requirements. The analyst then conducts an unannounced site visit to the facility and completes a variety of inspection activities as outlined in Figure 7.6 After the visit, the analyst prepares a report detailing any deficiencies, such as those listed in the text box. The department must then provide the report to the treatment facility. Facilities must submit written verification of correction for each deficiency to Health Care Services within 30 days.7 If the department determines that the facility has not corrected a deficiency by the deadline, it must assess a civil penalty of $250 to $500 per day, depending on the severity of the deficiency and with a maximum daily civil penalty of $1,000. This process for inspecting and notifying treatment facilities of deficiencies is a critical component of Health Care Services’ oversight and provides assurance that facilities are safe and meet the requirements of their licenses.
Examples of Possible Deficiencies
Class A deficiencies present an imminent danger to any resident of the facility.
Example: Lacking observation sheet for resident admitted for detoxification.
Class B deficiencies relate to the operation or maintenance of the facility and have a direct or immediate relationship to the health or safety of residents.
Examples: Missing documentation of health screening for residents or staff or lacking working light in bathroom.
Class C deficiencies relate to the operation or maintenance of the facility and have only a minimal relationship to the health and safety of residents.
Examples: Walls needing repainting or facility’s operations manual lacking certain reporting requirements.
Source: State regulations and Health Care Services compliance inspection documents.
We found that although Health Care Services’ compliance inspections were generally thorough, they were not always on time. We determined that the inspections we reviewed were thorough largely because the analysts maintained extensive and detailed inspection notes and identified in the associated reports both minor and significant deficiencies from their on‑site inspections of facilities and their reviews of facility paperwork. These notes show that the analysts reviewed the extensive requirements for maintaining a license, including those prescribed by state law, such as the facilities’ ensuring that they preserve resident confidentiality and employ staff trained in CPR and first aid if they offer detoxification services.
Although Health Care Services conducted all required inspections for the treatment facilities we reviewed, we found that the department did not conduct half of these inspections in a timely manner, as shown in Figure 8. This lapse left some facilities uninspected for years beyond the timeline set by state law. We selected treatment facilities and reviewed Health Care Services’ records to identify whether 26 facilities received on‑site inspections in fiscal years 2020–21 through 2022–23, and we analyzed whether Health Care Services followed its procedures for inspections.8 Health Care Services was able to demonstrate that all but two of the 26 treatment facilities had received an on‑site compliance inspection. One of the two facilities had received a virtual inspection. Health Care Services could not locate documentation for the other facility’s required inspection but noted that the department may have conducted a virtual inspection because that inspection was due during the pandemic. In addition, Health Care Services was late in conducting 12 of the inspections that became due after March 2020, the onset of the pandemic. Although not shown in Figure 8, Health Care Services had a median delay of 207 days for these 12 inspections. Two of the 12 inspections occurred within the time frame required in state law, which requires them to be completed during the two‑year licensure period, but those two inspections occurred outside the 90‑day inspection deadline in Health Care Services’ policy.
Figure 8
Health Care Services Conducted 12 of the 25 Applicable Compliance Inspections We Reviewed Late
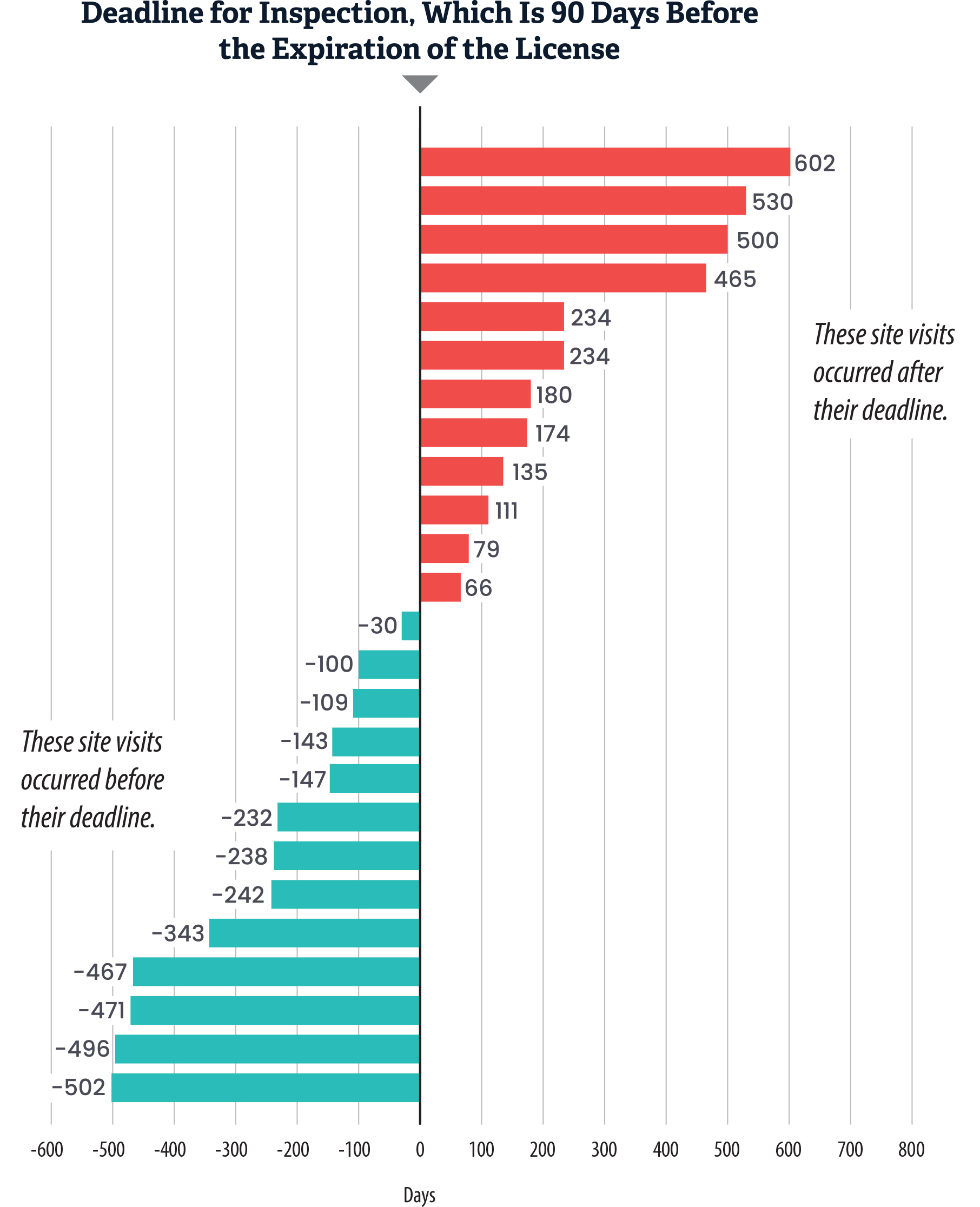
Source: Health Care Services inspection records from fiscal years 2020–21 through 2022–23.
Note: Of the 26 facilities we reviewed, 13 inspections were conducted before the deadline, and 12 were not. Health Care Services did not have any documentation for the inspection of the remaining facility; therefore, we could not determine whether it took place or whether it would have been late, and we were only able to assess the timeliness of inspections for 25 of the 26 facilities.
This bar chart visualizes the timeliness of compliance inspections conducted by Health Care Services from fiscal years 2020-21 through 2022-23, showing how many inspections occurred before or after the required deadline, which is 90 days before the expiration of the license. The Y-axis represents individual facilities, while the X-axis represents the number of days before or after the inspection deadline, with negative numbers indicating site visits that occurred before their deadline. The chart shows late inspections for 12 of the 25 we reviewed, with some inspections occurring 500 or more days late.
Because Health Care Services’ inspections can identify health and safety issues that may be detrimental to residents, it is important that the department conduct them on time. For example, one late inspection that we reviewed showed that some of the facility’s employees did not have proof of licenses to provide services and that some did not have required health screening paperwork, including evidence of a tuberculosis test. Another late inspection revealed moldy food and open food packages on the premises—deficiencies that could attract pests or risk contamination—and it also revealed that several residents did not have documentation of health screening paperwork. If Health Care Services does not conduct its inspections within its deadlines, similar or worse deficiencies may go unaddressed for significant periods.
Pandemic‑related restrictions and staffing shortages hampered Health Care Services’ operations for a number of years, leading to an inspection backlog. Health Care Services stated that it is difficult to quantify the scale of the backlog because of the sometimes unreliable inspection data resulting from problems in the system and staff data entry errors. Compliance inspections conducted before the pandemic were often punctual: of the eight applicable licenses we reviewed that expired in fiscal year 2020–21, seven licenses received their inspections in 2018 or 2019, which puts them well before the 90‑day deadline. After the beginning of the pandemic in 2020, Health Care Services did not conduct on‑site inspections through the middle of 2021, which created the inspection backlog.9
According to Health Care Services, numerous staff vacancies developed in the department when some staff did not feel comfortable conducting on‑site inspections because of infection concerns and others retired or transferred to other assignments. Our review of Health Care Services’ organizational chart showed that in December 2021, the department had six vacancies in its 34 compliance analyst positions, representing a vacancy rate of nearly 18 percent. Health Care Services has been working to address these vacancies. For example, Health Care Services confirmed to us that from January 2022 through June 2024, it hired 21 new analysts. Despite this hiring, Health Care Services had seven vacancies in its 34 compliance analyst positions as of May 2024, representing a vacancy rate of 21 percent, although none of these positions had been vacant for much more than a year. In August 2024, we spoke with some Health Care Services analysts who explained that caseloads, which had been difficult for some to manage during the pandemic, had returned to a sustainable level and that they felt they had the resources to properly manage their facilities. Health Care Services noted that it does not have a formalized caseload standard for its staff; rather, caseloads are based on the total number of facilities divided by the total number of analysts and fluctuate according to received applications and staff retention.
Health Care Services stated that as a result of its backlog, the department prioritized inspections of treatment facilities with higher risks, such as those offering detoxification services or those that had begun operations during the pandemic and had only received virtual inspections. Once Health Care Services resumed on‑site inspections, this prioritization likely increased the inspection delays for other facilities. Health Care Services also stated that many site visits were delayed when facilities reported having high occurrences of COVID‑19, requiring analysts to postpone visits until reported cases had subsided. Analysts generally need to plan inspections weeks in advance, so these sudden changes also affected the scheduling of inspections and the pace at which they could be completed.
Although Health Care Services has ended its pandemic on‑site inspection restrictions, the timeliness of the department’s inspections has also been limited by technological challenges. Health Care Services confirmed that its license database does not notify compliance analysts when inspections are coming due. This condition potentially creates the risk that some analysts miss key deadlines. Further, the database also does not currently generate reports for Health Care Services’ management about the timeliness of the department’s inspections. Each of these challenges necessitated that analysts maintain schedules of their own assigned facilities to maintain timeliness. However, Health Care Services reported that it began working with an external IT team in March 2024 to build and implement a new licensing system that will include mechanisms to notify analysts and supervisors about upcoming inspection due dates and provide reports about the overall pace and timing of its inspections. The department plans to launch this new system in 2025. Health Care Services also stated that it expects to fill nearly all compliance analyst vacancies by July 2025.
Health Care Services Does Not Always Promptly or Thoroughly Investigate Complaints
Key Points
- Health Care Services promptly began investigations of most high‑priority complaints, as it assigned 20 of the 24 high‑priority complaints that we reviewed to analysts within 10 days. However, Health Care Services consistently exceeded the 10‑day time frame required in state regulation for its investigations that were not designated high‑priority. Health Care Services took a median of over 100 days to assign the low‑ and medium‑priority cases we reviewed.
- During fiscal year 2022–23, Health Care Services improved its timeliness for completing investigations by an average of four months from its average completion time during fiscal year 2020–21. Overall, however, the department’s analysts took more than a year to complete 22 of the 60 investigations we reviewed that were conducted in fiscal years 2020–21 through 2022–23. This pace does not meet Health Care Services’ internal targets for analysts to complete investigations in 30 to 60 days.
- Health Care Services has not thoroughly investigated all allegations that unlicensed facilities are providing or advertising treatment services, nor has it always followed up, such as through conducting a site visit, to ensure that the unlawful behavior stopped.
- In the rare instances in which Health Care Services suspended or revoked a treatment facility license, such as for patterns of serious violations of regulations, we found that the department appropriately took action. Health Care Services suspended or revoked seven small facilities’ licenses during our audit period.
Health Care Services Does Not Assign Complaint Investigations to Staff Within the Required Time Frame
Health Care Services investigates three major categories of complaints about treatment facilities and investigates deaths, as the text box shows. More than half of the nearly 1,800 complaints that Health Care Services recorded receiving from fiscal years 2020–21 through 2022–23 were program complaints, such as complaints about a facility’s lack of cleanliness or alleging that unqualified staff are providing counseling services. Additionally, Health Care Services classifies complaints as high‑, medium‑, and low‑priority, according to the type and severity of the allegation contained in the complaint. For instance, Health Care Services would designate a complaint related to a refund request as a low‑priority program complaint, a complaint about program cleanliness or staff qualifications as medium‑priority, and the report of a resident’s death as high‑priority.
Four Types of Incidents That Health Care Services Investigates
Program Complaints
• General complaints against treatment facilities.
Unlicensed Facility Complaints
• Complaints against facilities that are advertising or providing treatment services without a valid license from Health Care Services.
Counselor Complaints
• Complaints against a counselor at a treatment facility.
Deaths
• Reported deaths of residents at licensed and certified facilities.
Source: Health Care Services complaints manual.
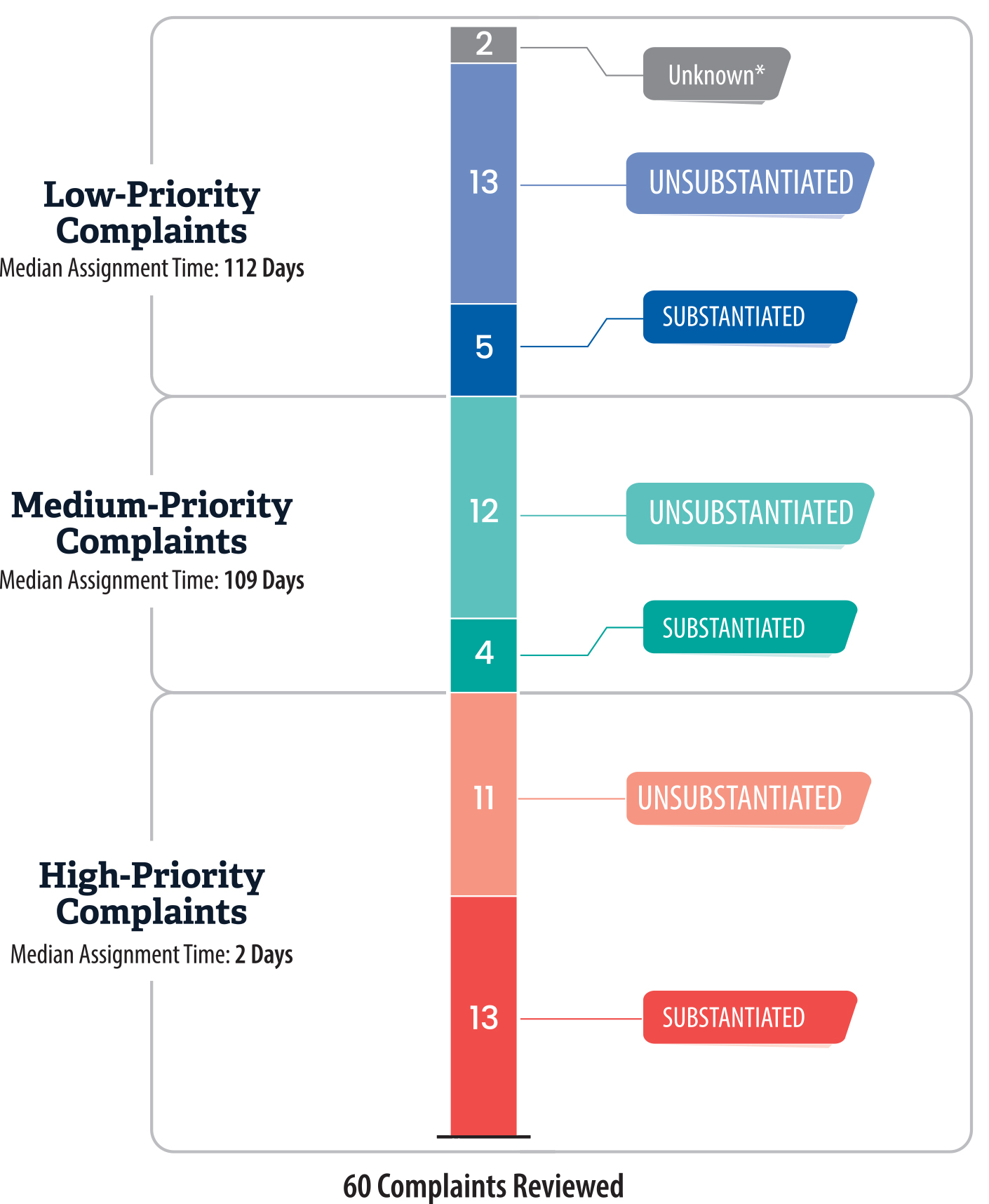
Although state regulations require Health Care Services to assign complaints to staff for investigation within 10 business days of receiving the complaint, the department frequently exceeded this time limit. Health Care Services refers to the staff members responsible for investigations as complaint analysts. We reviewed the investigations of 60 complaints, 37 percent of which were substantiated, to determine whether the complaints were assigned to analysts within the 10‑day limit. Among these 60 complaints, Health Care Services classified 20 complaints as low‑priority, 16 as medium‑priority, and 24 as high‑priority. As we show in Figure 9, more than one‑third of complaints that Health Care Services investigated were substantiated, regardless of the case priority type. We found that Health Care Services assigned only 24 of the 60 complaints, or 40 percent, to analysts within 10 days. Twenty of those 24 complaints were high‑priority, which had a median assignment time of two days, as Figure 9 also shows. Health Care Services more frequently exceeded the 10‑day limit for complaints that were not designated high‑priority. Specifically, Health Care Services took a median of 132 days—ranging from 26 days to 533 days—to assign the remaining complaints that were not assigned within 10 days, and 32 of these 36 complaints were designated as low‑ or medium‑priority.
Figure 9
Most of The Complaints We Reviewed Were Not Assigned to an Investigator Within 10 Days as Required, and More Than One‑Third Were Ultimately Substantiated
Source: Review of 60 Health Care Services complaints of the nearly 1,800 it received between fiscal years 2020–21 and 2022–23.
* We could not determine whether two complaints were substantiated. In one instance, the facility’s license expired shortly after Health Care Services received the complaint, so Health Care Services did not complete the investigation. In the other instance, Health Care Services had not yet completed the investigation at the time of our review.
This bar chart breaks down our review of 60 complaints received by Health Care Services into high-, medium-, and low-priority categories. The chart also shows the proportion of complaints that were substantiated versus unsubstantiated for each priority level. High-priority complaints (such as resident deaths or improper detoxification checks) were assigned to investigators more quickly, with a median assignment time of two days. In contrast, medium- and low-priority complaints took much longer to assign, with a median assignment of 109 and 112 days respectively.
According to Health Care Services, it did not consistently meet the 10‑day requirement for assigning complaint investigations because its staff misunderstood the requirement. When Health Care Services receives a complaint, Health Care Services policy directs staff to send a 10‑Day Letter to inform complainants that the department will review the complaint. Health Care Services explained that its staff believed that by opening the complaint internally and issuing this letter, they had satisfied the 10‑day requirement. However, we determined that issuing this letter does not meet the requirement because Health Care Services does not assign an investigator to the complaint. We met with Health Care Services’ legal counsel, who agreed that issuing the letter does not satisfy the requirement. In response to our conversations during this audit, Health Care Services recognized that it had misinterpreted the requirement, and the department plans to update its processes to ensure that it assigns complaint investigations to analysts within 10 days. Health Care Services also plans to update its written policies and provide staff training on the new process.
Although Health Care Services did not always assign complaints to investigators within the 10‑day requirement, it more frequently met this requirement for high‑priority complaint investigations because it prioritized investigating complaints that posed a greater threat to resident safety. Health Care Services identifies several types of complaints as high‑priority, and we provide more detail about these complaints in the text box. Health Care Services assigned seven of the 10 death investigations we reviewed within one day, two within eight days, and assigned the remaining investigation, which involved a non‑resident death, much later.10 Health Care Services explained that it took significantly longer to assign the lower‑priority complaints because it did not prioritize beginning investigations of complaints that posed a lower threat to resident safety.
Examples of High‑Priority Complaint Allegations
• Resident deaths
• Insufficient detoxification checks
• Problems with managing resident medications
• Sexual misconduct
Source: Health Care Services complaints manual.
Health Care Services also met the 10‑day requirement for each of the counselor complaints that we reviewed. Health Care Services considers most types of complaints about counselors to be high‑priority because of the serious threat to residents these allegations present. Examples of counselor complaints include allegations of an inappropriate relationship with a resident or of falsifying documents such as counseling notes. Among our selection of 60 complaints, we reviewed 10 complaints against counselors, and we found that Health Care Services assigned these investigations in an average of two days. Generally, delays in assigning complaints to analysts contribute to overall delays in completing investigations, which we discuss in the next section.
Health Care Services’ Complaint Investigation Reports Are Submitted Several Months After Target Due Dates
State law does not contain a completion deadline for complaint investigations—such as those in response to deaths or allegations of unlicensed treatment facilities—but Health Care Services has established some internal deadlines. According to state law, Health Care Services’ death investigation policy shall ensure that the department investigates a resident’s death “in a timely manner,” but the law does not define the term timely. Health Care Services has established a policy that states that complaint analysts should submit all investigative reports to their supervisor no later than 30 to 60 calendar days after a site visit or after the date of assignment for investigations without a site visit, depending on the type of complaint. Heath Care Services complaint analysts complete the activities listed in the text box, among other activities applicable to the investigation, and they create a report that includes their investigative findings and a determination regarding whether a complaint allegation is substantiated. Health Care Services’ policies do not include other timeline requirements for investigations after the submission of the report to the supervisor, with the exception of some internal interim deadlines for counselor complaint investigations.
Key Complaint Investigation Activities
• Obtain and review facility documentation relevant to the complaint.
• Conduct staff and resident interviews.
• Complete on‑site visits.
• Review security footage.
• Analyze applicable statutes, regulations, and certification standards.
Source: Health Care Services complaints manual and investigation reports.
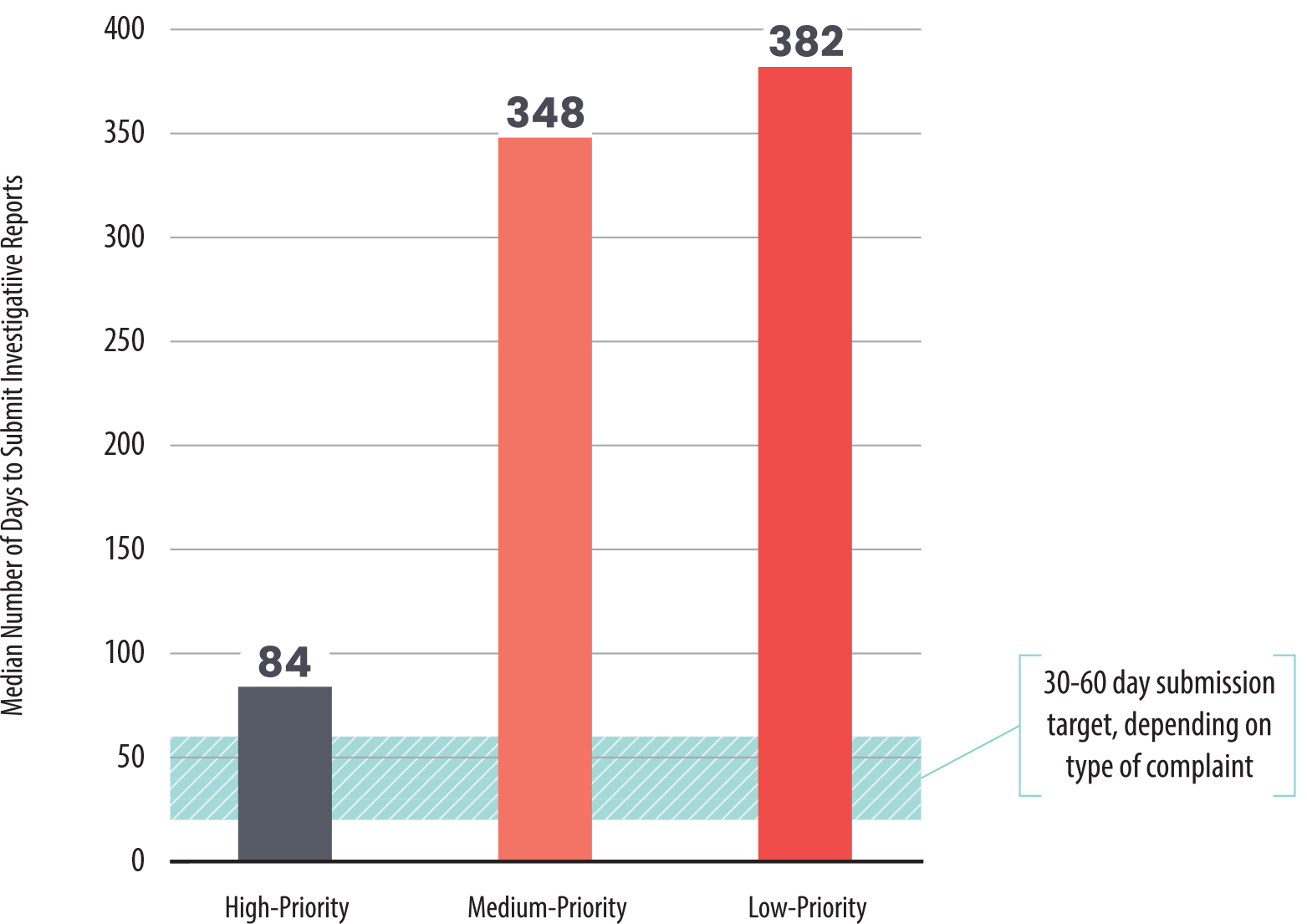
Of the 60 complaints we discuss in the previous section, we found that Health Care Services’ complaint analysts generally completed high‑priority investigations in a median of 84 days. In contrast, the department’s analysts took more than three times as long—more than 300 days—to submit investigative reports for medium‑ and low‑priority investigations, as we show in Figure 10. Health Care Services’ analysts took more than a year to submit reports for 22 of the 60 investigations we reviewed. In ten of those investigations, the analysts took more than 600 days to complete their investigation reports, and three of these investigations took more than 800 days.
Figure 10
Health Care Services Takes Significantly Longer to Submit Investigative Reports Than Its Target Due Dates
Source: Selection of 60 Health Care Services complaint investigation files from fiscal years 2020–21 through 2022–23.
This bar chart compares the time it took Health Care Services to submit investigative reports for high-, medium-, and low-priority complaints between fiscal years 2020–21 and 2022–23. The X-axis shows the priority level, and the Y-axis shows the number of days from complaint assignment to report submission. High-priority investigations were completed in an median of 84 days, while medium- and low-priority cases took more than three times as long, often exceeding 300 days.
We found that delays in conducting complaint investigations can potentially put resident safety at risk. For example, in one of the investigations that took more than 800 days, Health Care Services did not find evidence to substantiate the initial allegation. However, the complaint analyst found evidence during the investigation of the facility having expired food, not destroying the medication of discharged residents, and altering prescription medication labels. Health Care Services determined that these actions and conditions violated state regulations. The facility—which had an active license as of August 2024 that has now expired—corrected one of the deficiencies that Health Care Services identified during the site visit, and Health Care Services later reported that the facility had corrected the other deficiencies. Nonetheless, when it does not complete investigations within the required time frame, Health Care Services cannot identify problems promptly, ensure that facilities take appropriate corrective actions, and safeguard the health and safety of residents.
Health Care Services’ extended completion time for some investigations is caused primarily by delays in assigning investigations to analysts and, according to Health Care Services, by the complexity of investigating high‑priority complaints. As we previously discussed, Health Care Services misinterpreted the requirement for assigning complaint investigations within 10 days, and this delay in assigning investigations often exceeded the 10‑day time frame for lower‑priority complaints. In fact, in the previously described example in which the investigation took more than 800 days, the department took nearly a year to assign the complaint. The department asserts also that high‑priority complaints, such as those involving deaths, detoxification checks, and sexual misconduct, require significant staff time to thoroughly investigate to identify any threats to the health and safety of residents. This could contribute to delays in beginning other investigations.
Nonetheless, Health Care Services has modestly improved the timeliness of its complaint investigations over the course of the audit period, in part because the department increased staffing in its complaints section by 20 percent. As we show in Figure 11, Health Care Services completed investigations about four months faster in fiscal year 2022–23 than it did in fiscal year 2020–21. However, this improvement in completion time is still at least seven months later than the targeted due date. In 2022, Health Care Services added four analysts to its staff of 20 complaint analysts. The department stated that the purpose of these positions was to enable the complaints unit to more promptly assess and investigate complaints, especially in Southern California. Health Care Services also explained that it improved its tracking of case assignment and closure dates in early 2021, which allowed supervisors to identify complaint investigations that had been open for an extended period. In addition, Health Care Services stated that it intends to improve its timeliness in completing lower‑priority investigations through increased supervisor monitoring and communication with analysts and streamlined report templates.
Figure 11
Despite Some Recent Improvement, Health Care Services Submits Complaint Investigations Several Months After Its Targeted Due Dates
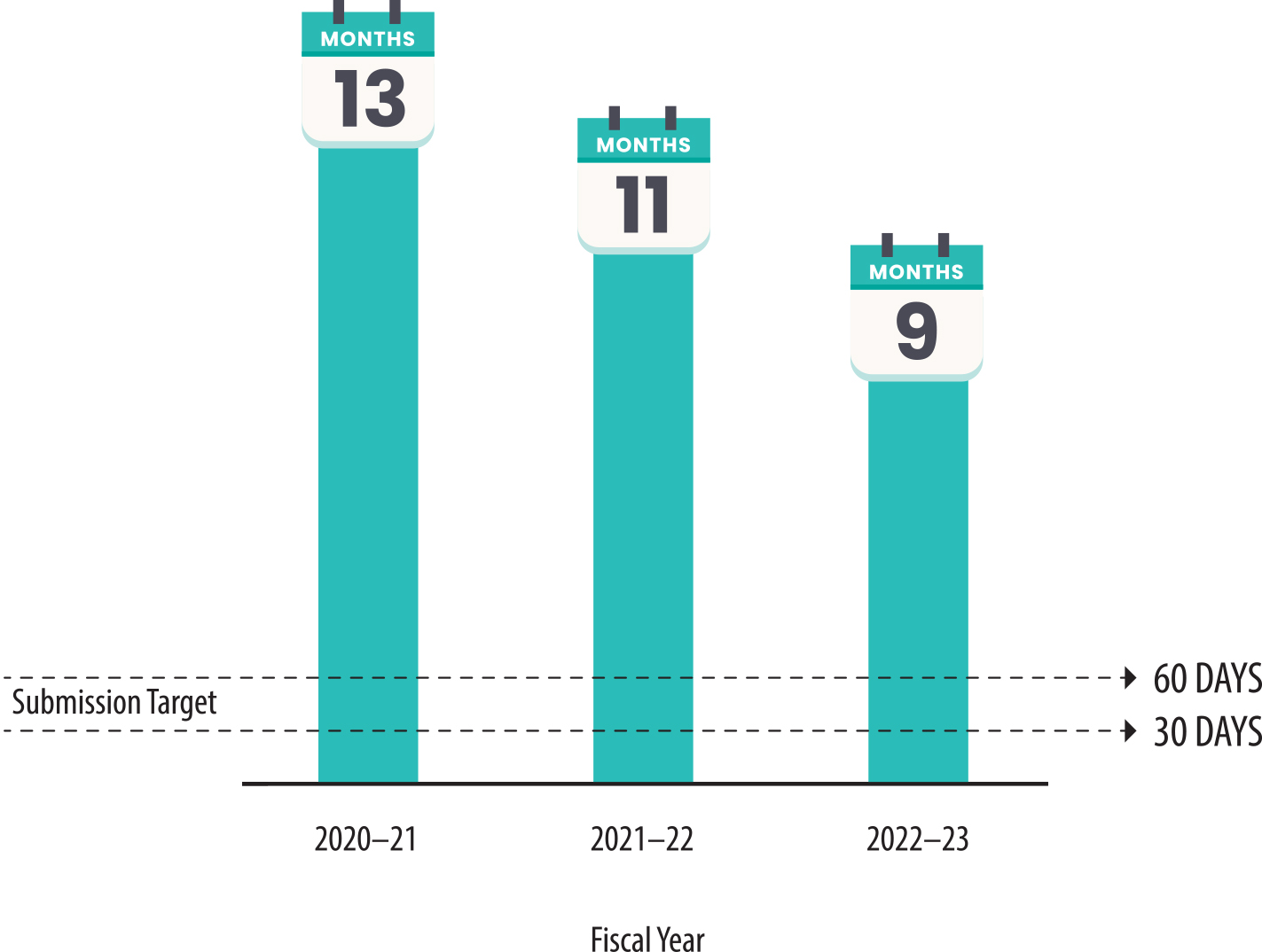
Source: Selection of 60 Health Care Services complaint investigation files from fiscal years 2020–21 through 2022–23.
This bar chart shows the months it took Health Care Services to submit complaint investigation reports from 2020-21 through 2022-23, comparing actual completion times with targeted due dates. The X-axis represents fiscal years, and the Y-axis represents the number of months taken to complete investigations. The graph shows an improvement in timeliness by fiscal year 2022–23, with an average of nine months, compared to 11 months in fiscal year 2021-22, and 13 months in fiscal year 2020-21.
Health Care Services Has Not Adequately Investigated Complaints About Certain Unlicensed Treatment Facilities
State law prohibits any drug and alcohol treatment facility that provides recovery, treatment, or detoxification services from operating without first obtaining a valid license from Health Care Services. According to state law, if a treatment facility is alleged to be providing treatment services without a license, Health Care Services must conduct a site visit to investigate these allegations. State regulations further prohibit facilities without a valid license from advertising that they are providing treatment services. According to Health Care Services’ policy, the department must conduct an investigation into all allegations of unlicensed facilities providing or advertising treatment services. If the department concludes that allegations related to unlicensed treatment facilities are substantiated, Health Care Services must notify the facility of its findings and inform it of potential civil action if the facility continues to provide services beyond the date specified in the notice. For example, Health Care Services will issue a Notice of Operation in Violation of Law (notice of violation) to the facility, directing the facility to cease providing or advertising services that require a license, as we show in Figure 12.
Figure 12
Health Care Services’ Process for Sanctioning Unlicensed Facilities Can Involve Civil Penalties
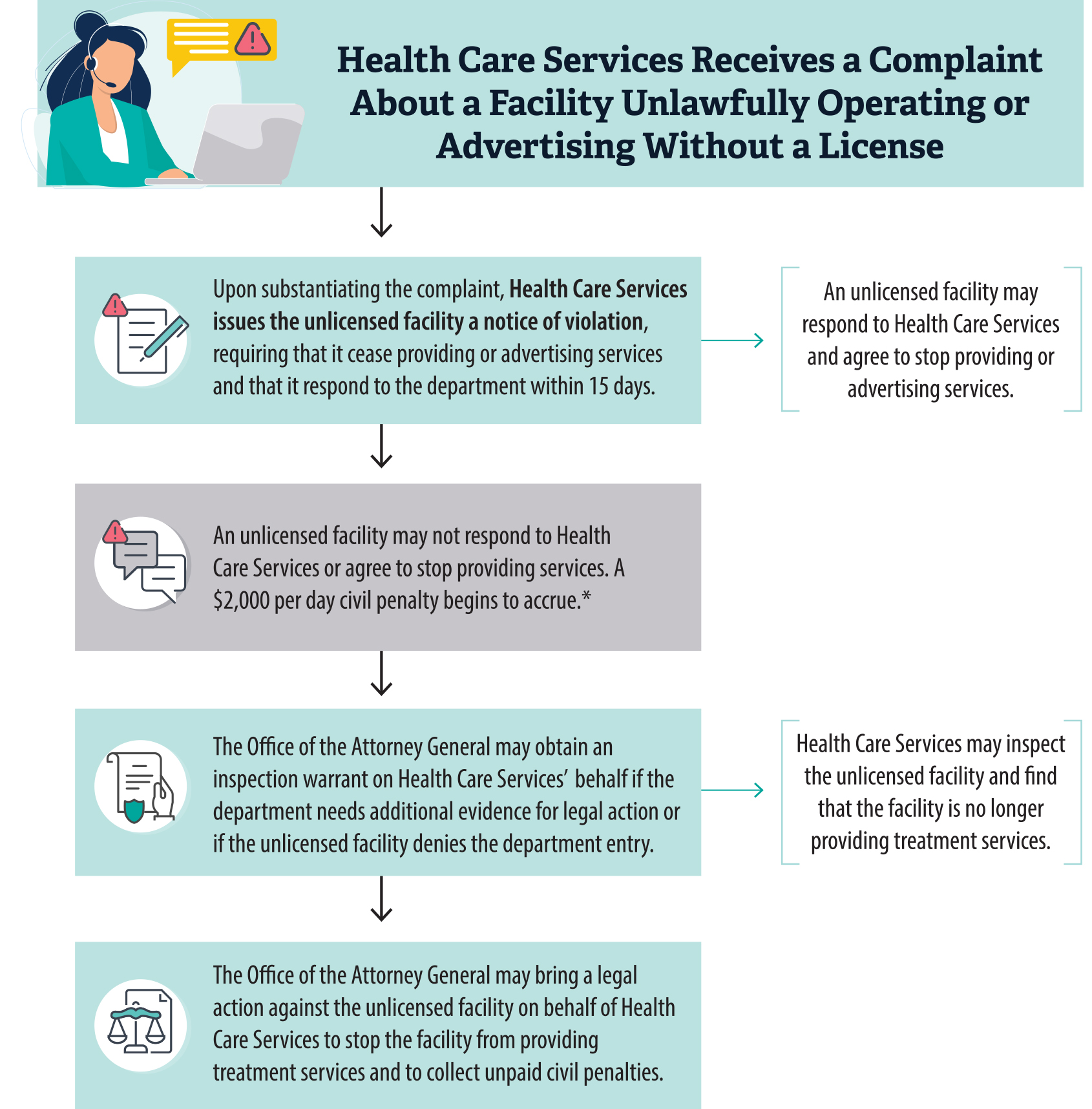
Source: State law and Heath Care Services complaint manual.
* Health Care Services does not seek civil penalties when unlicensed facilities are advertising licensable services as long as Health Care Services does not find that the facilities are providing such services.
This flowchart outlines the process Health Care Services follows when investigating unlicensed treatment facilities suspected of providing or advertising services unlawfully. It starts with the receipt of a complaint, followed by an investigation, site visits, and the issuance of notices of violation if the facility is found to be non-compliant. The chart also shows follow-up steps, such as additional inspections and legal actions if necessary.
We reviewed all 15 complaints related to unlicensed facilities between fiscal years 2020–21 and 2022–23 that resulted in a notice of violation, and we determined that Health Care Services did not consistently perform site visits when it conducted investigations into the allegations. Specifically, the department performed site visits for just seven of the 15 complaints, despite having a policy guideline to perform site visits for all unlicensed facility complaints. Health Care Services explained that it did not conduct the site visits for the other eight complaints because of its pandemic‑related travel restrictions. However, we expected Health Care Services to return to the facilities as soon as travel restrictions were lifted in late 2021 to conduct the site visits it had foregone and to confirm that these unlicensed facilities were indeed not unlawfully providing services.
When Health Care Services substantiated allegations of unlicensed facilities advertising services that they were not licensed to provide, the department did not always verify the facilities’ claims that they were not, in fact, providing the services. Of the 15 investigations of unlicensed facilities that we reviewed in which Health Care Services had substantiated an allegation, the department determined that 11 of these unlicensed facilities were advertising services that required a license. For six of the 11 facilities, Health Care Services did not verify facilities’ statements that they only advertised for but did not provide services that required a license. Health Care Services could have verified those statements by conducting site visits to ensure the facilities’ statements were indeed true. The department stated that it did not visit the facilities because after the facilities had removed the advertisements, they were no longer in violation of state law, and thus no site visit was needed. However, we disagree: if an unlicensed treatment facility was in fact providing services that require a license, such as detoxification, Health Care Services would be unable to detect such activity without conducting a site visit. Health Care Services can provide greater assurance that these unlicensed facilities are not providing services: the department could consistently conduct a site visit whenever a facility advertises that it provides treatment services but does not have a license.
We also found that even after Health Care Services substantiated allegations, the department did not always perform adequate follow‑up to ensure that the unlicensed facilities stopped advertising or providing services that they cannot provide without a license. Of the 15 investigations of unlicensed facilities that we reviewed in which Health Care Services had substantiated an allegation for providing or advertising services, it found that four of the unlicensed facilities were providing treatment services illegally. In two of these investigations, Health Care Services appropriately engaged with its legal counsel to consider next steps, including assessing civil penalties. For example, one complaint we reviewed resulted in the facility accruing $104,000 in civil penalties as of March 2024. However, for the other two investigations, we found no indication that Health Care Services conducted follow‑up, such as through site visits, and we thereby found insufficient evidence that the unlicensed facilities ceased providing treatment services they are not allowed to provide. In both of these latter instances, Health Care Services’ investigations occurred during its pandemic travel restrictions, so the analysts did not conduct site visits. One of these facilities wrote to Health Care Services to state that it was currently operating mostly as a sober living home, but Health Care Services did not demonstrate that it verified the veracity of this claim.
State regulations clarify that Health Care Services may conduct another site visit to verify that the unlicensed facility is no longer in violation of state law, but the department did not do this in either instance. Health Care Services has not been able to explain why it did not follow up with these facilities after it began conducting site visits again. However, the department also stated that it would open new investigations for both of these unlicensed facilities, and conduct site visits as soon as possible. When Health Care Services does not follow up to ensure that unlicensed facilities cease operations, those facilities may continue to provide potentially hazardous services to residents without any assurance of the qualifications of its staff and the safety of residents and their environments.
Health Care Services Appropriately Suspends or Revokes Treatment Facility Licenses When It Identifies Patterns of Serious Violations
State law allows Health Care Services to suspend or revoke the license of any treatment facility that violates state law or regulations. For example, state regulations permit Health Care Services to seek a suspension or revocation of a license when it issues a notice of deficiency for any action that has resulted in death, serious physical harm, or imminent danger to a resident of a facility. After Health Care Services issues this notice, it can temporarily suspend the facility’s operations if necessary to protect residents from harm. The temporary suspension remains in effect until Health Care Services schedules a hearing and makes a determination regarding whether to revoke the facility’s license. A facility has 15 days from the date it is notified of the temporary suspension to file a defense. Health Care Services may also seek a suspension or revocation depending on how facilities follow up with different types of deficiencies that the department identifies, which the text box describes. If a facility fails to correct any Class A deficiency by the specified date in the notice or repeatedly fails to correct Class B deficiencies, or if the license holder fails to pay civil penalties, Health Care Services can proceed with suspending or revoking that facility’s license. Facilities may agree to terms with Health Care Services, and most suspensions end in settlement agreements. Health Care Services may still revoke a facility’s license through a settlement agreement, but the department and a facility may also agree to terms that allow a facility to keep its license.
Types of Deficiencies
Class A: Issues that present imminent danger to residents at a facility, such as or physical injury.
Class B: Issues with the operation or maintenance of a facility that have a direct or immediate relationship to the health or safety of facility residents.
Class C: Issues with the operation or maintenance of the facility that have only a minimal relationship to the health or safety of facility residents.
Source: State regulations.
Our review found that Health Care Services is appropriately suspending and revoking treatment facility licenses. In addition, according to Health Care Services’ policy, the department monitors the potential need for suspension and revocation by assessing patterns of complaints or deficiencies. To verify that Health Care Services performs this monitoring, we reviewed facilities with high‑priority complaints in Health Care Services’ log of complaint allegations to determine whether any of these facilities demonstrated a pattern of serious complaints. For example, we found that Health Care Services investigated three high‑priority complaints related to a single facility between July 2020 and June 2023. Health Care Services did not substantiate the allegation related to one of the three complaints, but it identified multiple Class A, B, and C deficiencies in the course of the investigations into the other two complaints. Nonetheless, Health Care Services’ documentation shows that the facility corrected these deficiencies in a timely manner, and Health Care Services did not repeatedly cite the facility for the same deficiencies. Because the facility corrected the deficiencies and did not pose a current or ongoing threat to the health and safety of residents, Health Care Services did not seek to suspend or revoke the facility’s license.
We identified seven licenses that Health Care Services suspended between fiscal years 2020–21 and 2022–23, and it ultimately revoked four of those suspended licenses. When Health Care Services suspended or revoked these licenses, the department had determined that the treatment facility posed a significant threat to resident health and safety. We provide examples of such threats in the text box. For example, in one instance a facility did not conduct required observation checks every 15 minutes for a depressed resident at risk of self‑harm, as required by the facility’s policies and procedures. This facility instead falsified its records of the checks and did not check on a resident for over an hour and a half, during which time the resident died by suicide. Health Care Services’ investigation determined that the facility’s conduct would continue to place residents at risk of physical or mental abuse or abandonment, and it appropriately revoked the facility’s license.
Examples of Causes for Suspension or Revocation
• Failure to conduct adequate health checks on residents detoxifying from drugs or alcohol.
• Provision of certain services without Health Care Services’ prior authorization.
• Failure to refer patients to a higher level of care, such as psychiatric treatment, when necessary.
Source: Health Care Services complaint investigation files and court filings.
Six of the seven license suspensions that we reviewed involved a treatment facility’s repeatedly violating licensing regulations. Such license suspensions indicate that Health Care Services is actively monitoring and suspending the licenses of problematic facilities. Health Care Services may begin the process of suspending or revoking a license after it has identified severe deficiencies during compliance inspections or complaint investigations. When Health Care Services initiates a program complaint or death investigation, analysts review any past complaints at that facility. Health Care Services’ policy is to begin discussions about whether the facility’s actions warrant a license suspension as soon as it identifies a pattern of misconduct. For example, during an investigation of a facility in October 2021, Health Care Services identified multiple deficiencies, such as providing detoxification and incidental medical services without Health Care Services’ approval, improperly storing and tracking resident medication, and staff providing counseling services without having proper credentials. In total, Health Care Services identified one Class A deficiency, seven Class B deficiencies, and eight Class C deficiencies at the facility.
In December 2021, the facility administrators corrected each of the deficiencies, which Health Care Services approved. However, Health Care Services conducted another investigation after the same facility reported a resident’s death in March 2022. During the investigation, Health Care Services identified and substantiated multiple new deficiencies, such as failing to have two staff on duty at all times, failing to maintain a drug‑free environment, and failing to provide adequate counseling services. Health Care Services suspended this facility’s license because of the facility’s repeated violations and its failure to protect the health and safety of its residents. The seventh license suspension we reviewed related to inadequate observation checks and falsified documents, as we previously described, and resulted in a resident’s suicide at the facility. This suspension did not require prior violations before Health Care Services began the suspension and revocation process.
Finally, we found that Health Care Services ensured that owners with suspended or revoked licenses did not obtain licenses for new facilities against the terms of their suspension or revocation. State law permits Health Care Services to terminate review of an application for licensure from any person or entity whose license was previously suspended or revoked for a period of five years from the date of the final decision and order. For each of the facilities with suspended and revoked licenses, we reviewed Health Care Services’ records to identify any instances in which those facility operators obtained a new license or renewed a license after the date of suspension or revocation. When we compared data on these operators to the moratorium period that Health Care Services established, we found that none of the applicable operators received a new license or license renewal against orders from Health Care Services during that period.
Other Area We Reviewed
To address the audit objectives approved by the Joint Legislative Audit Committee, we also reviewed whether Health Care Services evaluates the effectiveness of treatment and patient care at facilities.
Effectiveness of Treatment at Licensed Facilities
Health Care Services does not measure the effectiveness of residential treatment facilities, and state law does not require it to do so. State law related to the licensing of treatment facilities does not require Health Care Services to evaluate the effectiveness of those facilities. We evaluated whether Health Care Services could reasonably measure the effectiveness of licensed treatment facilities, such as by using available Medi‑Cal survey data related to substance use programs, but we did not identify any feasible methods. Specifically, we found that although Health Care Services has access to survey data from University of California, Los Angeles’s Integrated Substance Abuse Programs, the data are unlikely to yield meaningful conclusions on the effectiveness of residential treatment. Specifically, the data largely pertain to facilities that are not licensed by Health Care Services, such as outpatient facilities for drug and alcohol rehabilitation. Further, the survey data are collected exclusively from current residents and therefore has limited information on their outcomes after treatment.
It is generally difficult to measure the effectiveness of alcohol and substance use treatment, in part because of the nature of alcohol and substance use disorders. An individual’s specific substance use problems will affect the person’s needs for services, the services that may be effective, and the effectiveness of those services in addressing the individual’s substance use disorder. Other factors, such as the individual’s social environment and the duration of the individual’s substance use, also play key roles in the effectiveness of any particular treatment. Outside studies on the effectiveness of residential drug and alcohol use treatment, such as those conducted by the Canadian Agency for Drugs and Technologies in Health, similarly cite the difficulty of evaluating the effectiveness of treatments. American Addiction Centers, which states that it operates a large nationwide network of treatment facilities, identified metrics such as the number of individuals who complete a program and the percentage of individuals who remain sober after treatment as potential measures of effectiveness. However, American Addiction Centers also notes the challenges of evaluating the effectiveness of treatment programs and, as we describe above, other factors that would be out of the control of a treatment facility that can influence the success of treatment programs, such as an individual’s educational and employment background. Finally, we reviewed states that provide oversight of treatment facilities similar to that of Health Care Services: Florida, New York, Oregon, Texas, and Washington. We found that these states likewise do not measure the effectiveness of treatment facilities.
Recommendations
Legislature
If the Legislature seeks to address concerns about the overconcentration of treatment facilities in residential communities, it could potentially enact legislation to address the issue without violating federal housing and disability law. In one possible example, the Legislature could require Health Care Services to issue regulations under the Administrative Procedure Act for making specific findings of fact when a license applicant’s proposed facility will result in overconcentration and for denying and imposing additional requirements on the license when overconcentration would create an institutional setting for residents or impede their integration into the community. In another possible example, the Legislature could amend the statutory exemption from local zoning regulations to exclude new licensees that will effectively have more than six residents, such as closely located facilities that will share owners, directors, and amenities, and have more than six residents in total among them.
Health Care Services
To ensure that it inspects all treatment facilities as required by state law, Health Care Services should do the following by October 2025:
- Provide management with information about the timeliness of compliance inspections. Such reporting should include the license expiration date for each facility, the status of the inspections, and the date by which the inspections must be completed to meet the 90‑day inspection target.
- Implement a mechanism in its licensing database that notifies its staff of the dates for upcoming compliance inspections for their caseload so they can plan accordingly.
- Fill its vacant positions.
To ensure that it assigns complaints to analysts for investigation within 10 days as required by regulations, Health Care Services should update its policies and staff training by April 2025 to clarify the requirement.
To improve the timeliness of its investigations and align investigations of counselor complaints, which have interim deadlines for key investigation steps, with other complaint types, Health Care Services should implement guidelines by October 2025 that specify the length of time analysts should take to complete key steps in the investigation process for different types of investigations.
To ensure that it is conducting thorough investigations of unlicensed treatment facilities, Health Care Services should conduct site visits beginning in December 2024 in all instances in which there is an allegation that an unlicensed facility is advertising or providing treatment services without a license.
To ensure that unlicensed treatment facilities do not continue to provide services without a license after an investigation substantiates the allegation, by April 2025, Health Care Services should develop and implement a follow‑up procedure, such as by performing another site visit, to confirm the unlicensed facility has ceased providing such services.
We conducted this performance audit in accordance with generally accepted government auditing standards and under the authority vested in the California State Auditor by Government Code section 8543 et seq. Those standards require that we plan and perform the audit to obtain sufficient, appropriate evidence to provide a reasonable basis for our findings and conclusions based on the audit objectives. We believe that the evidence obtained provides a reasonable basis for our findings and conclusions based on our audit objectives.
Respectfully submitted,
MIKE TILDEN, CPA
Chief Deputy State Auditor
October 24, 2024
Staff:
Jim Adams, MPP, Audit Principal
Nicole Madera, MPP, Senior Auditor
Brian D. Boone, CIA, CFE, Senior Auditor
Dominik Baer
Michael Henson
Daniella Jacobs
Kaleb Knoblauch
Data Analytics:
Ryan Coe, MBA, CISA
R. Wade Fry, MPA
Aren Knighton, MPA
Lily Nuñez, MPP
Legal Counsel:
Joe Porche
Appendices
Appendix A
Geographic Distribution of Licensed and Certified Treatment Facilities as of August 2024
Treatment facilities licensed or certified by Health Care Services are located throughout the State. However, there are concentrations of facilities in certain regional population centers. Using the map of licensed and certified facilities that Health Care Services publishes on its website, we created the following figures to depict the geographic distribution of treatment facilities across the State. Figure A.1 shows the distribution of licensed or certified treatment facilities across California, regardless of facility capacity. To provide further local context, Figure A.2 and Figure A.3 show the distribution of licensed or certified treatment facilities in Orange County. Figure A.4 shows the distribution of licensed or certified treatment facilities in Ventura County. Each map includes both residential facilities that are licensed or licensed and certified and outpatient facilities that only hold a certification.
Figure A.1
Map of Licensed or Certified Substance Use Disorder Treatment Facilities in California
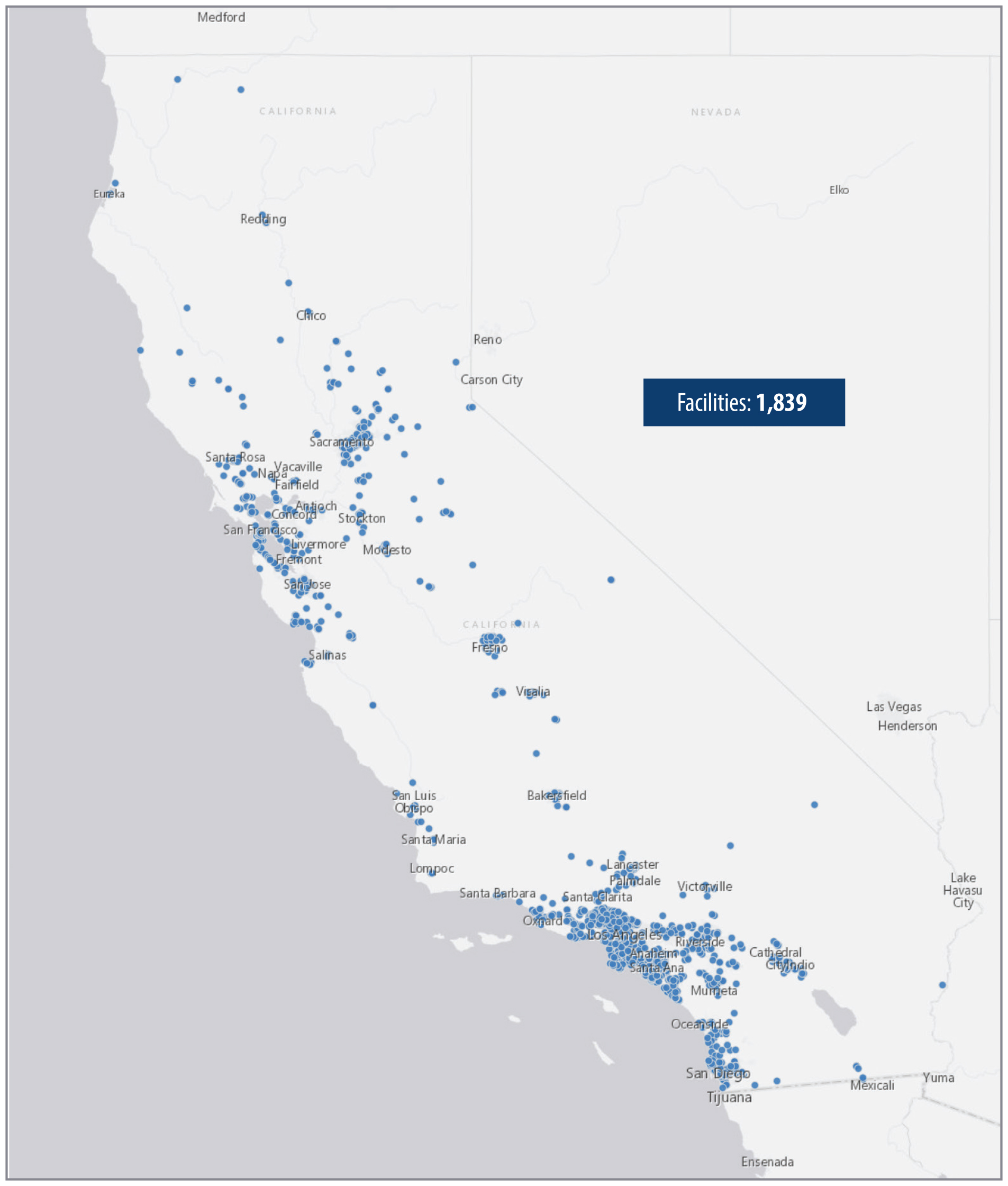
Source: Health Care Services map of substance use disorder treatment facilities.
This is a statewide map of California showing the geographic distribution of licensed or certified substance use disorder treatment facilities. Each dot represent a facility, showing that Southern California has more clusters of facilities compared to northern and rural regions.
Figure A.2
Map of Licensed or Certified Substance Use Disorder Treatment Facilities in Southern Orange County
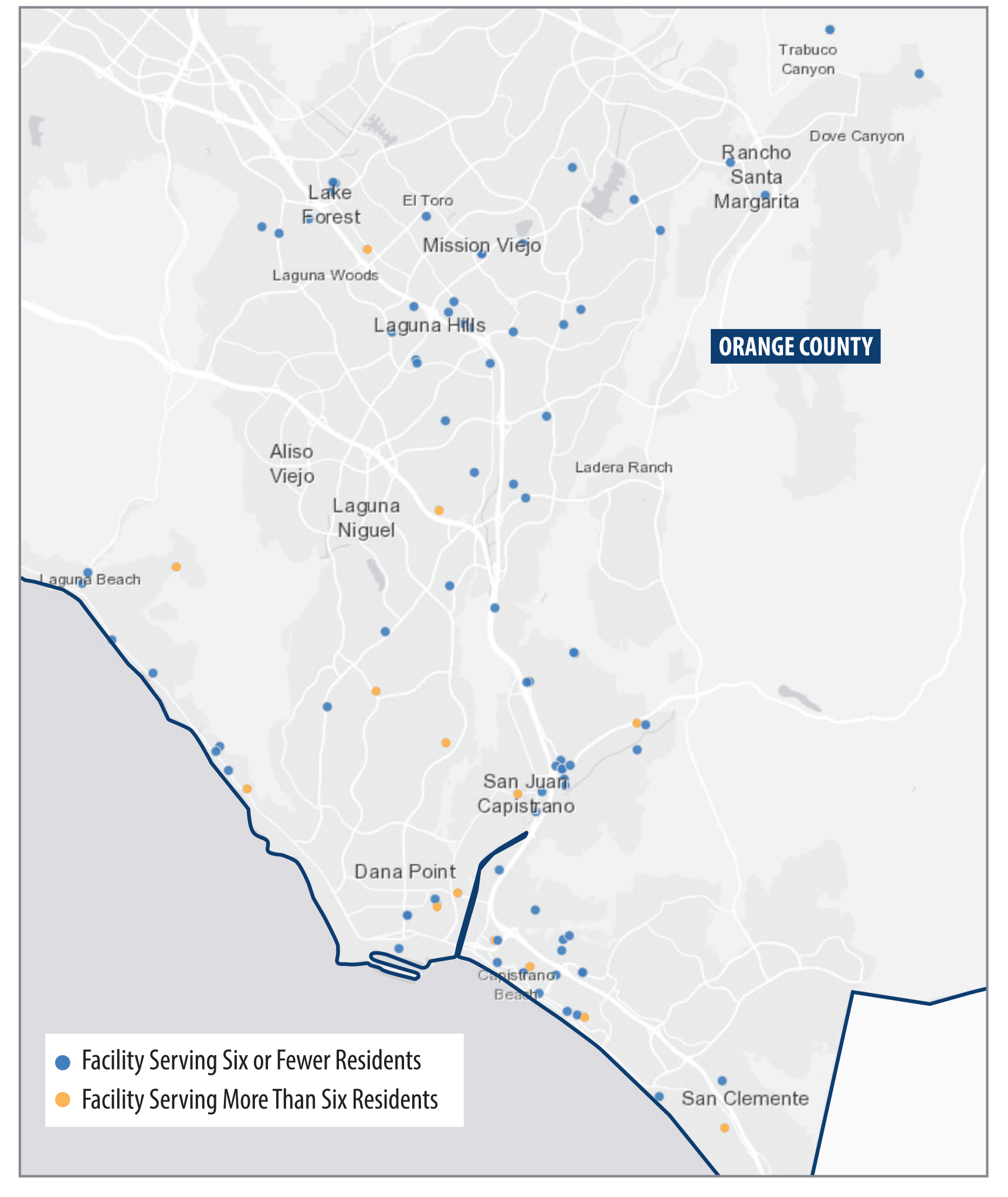
Source: Health Care Services map of substance use disorder treatment facilities.
This map highlights the geographic distribution of licensed or certified substance use disorder treatment facilities located in Southern Orange County, California. Facilities are categorized by their size, with a color indicating whether they serve six or fewer residents or more than six residents.
Figure A.3
Map of Licensed or Certified Substance Use Disorder Treatment Facilities in Northern Orange County
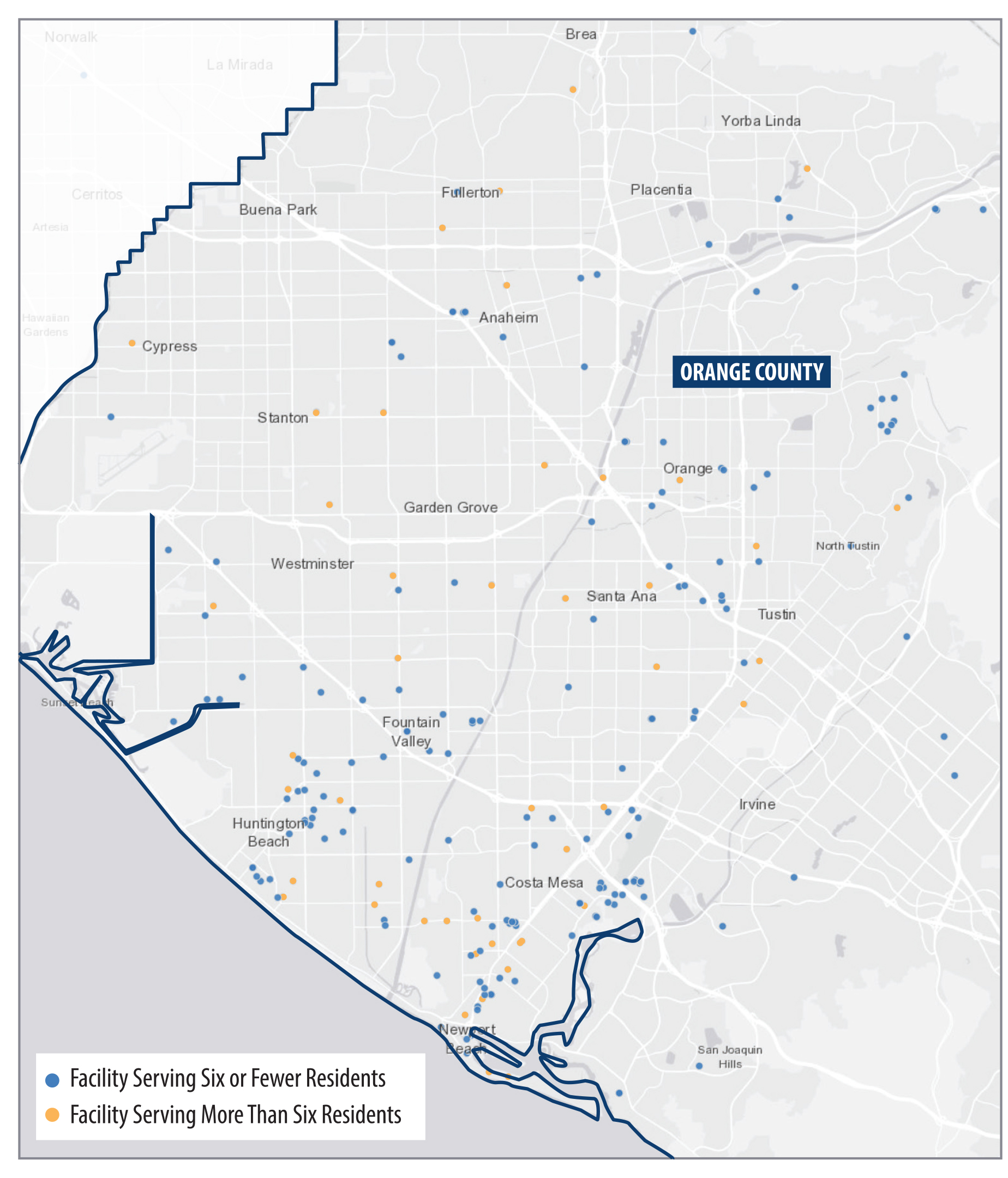
Source: Health Care Services map of substance use disorder treatment facilities.
This map shows the distribution of licensed or certified substance use disorder treatment facilities in Northern Orange County, California. Similar to Figure A.2, the facilities are categorized by size, and the map shows clusters of facilities in certain neighborhoods.
Figure A.4
Map of Licensed or Certified Substance Use Disorder Treatment Facilities in Ventura County
Source: Health Care Services map of substance use disorder treatment facilities.
This map shows the distribution of licensed or certified substance use disorder treatment facilities in Ventura County, California, categorized by facility size. The map shows concentrations of facilities in more populous areas like the city of Ventura, Camarillo, and Thousand Oaks.
Appendix B
Scope and Methodology
The Joint Legislative Audit Committee (Audit Committee) directed the California State Auditor to conduct an audit of Health Care Services related to its oversight of licensed recovery and treatment facilities. Table B lists the objectives that the Audit Committee approved and the methods we used to address them. Unless otherwise stated in the table or elsewhere in the report, statements and conclusions about items selected for review should not be projected to the population.
Response
California Department of Health Care Services
October 7, 2024
THIS LETTER SENT VIA EMAIL
Grant Parks
California State Auditor
621 Capitol Mall, Suite 1200
Sacramento, CA 95814
RE: RESPONSE TO DRAFT AUDIT REPORT 2023-120
Dear Mr. Parks:
The Department of Health Care Services (DHCS) hereby submits the enclosed response to the California State Auditor (CSA) draft audit report number 2023-120, titled, “Drug and Alcohol Treatment Facilities Are Sometimes Concentrated In Residential Areas, As Allowed, But State Oversight Is Not Always Timely or Thorough”.
In the above draft audit report, CSA issued five recommendations for DHCS. DHCS has reviewed all of CSA’s recommendations and has prepared a response describing the nature of the corrective actions taken or planned.
DHCS is committed to robust oversight of residential treatment facilities to ensure Californians receive safe and high-quality care. In addition to proactively implementing initial operational changes to improve outcomes, the 2023-24 Budget included additional resources to strengthen our compliance oversight, including additional positions for a statewide presence and increasing fees to strengthen DHCS’ capacity to conduct oversight and enforcement to ensure patient safety and quality care.
DHCS appreciates the work performed by CSA and the opportunity to respond to the draft audit report. If you have any questions, please contact DHCS’ Office of Compliance, Internal Audits at (916) 261-0346.
Sincerely,
Michelle Baass
Director
Enclosure
cc:
| cc: Erika Sperbeck Chief Deputy Director Policy and Program Support Department of Health Care Services Erika.Sperbeck@dhcs.ca.gov | Saralyn Ang-Olson, JD, MPP Chief Compliance Officer Office of Compliance Department of Health Care Services Saralyn.Ang-Olson@dhcs.ca.gov |
| Sarah Books Chief Deputy Director Health Care Programs Department of Health Care Services Sarah.Brooks@dhcs.ca.gov | Wendy Griffe, MPA Chief Internal Audits Department of Health Care Services Wendy.Griffe@dhcs.ca.gov |
| Tyler Sadwith State Medicaid Director Department of Health Care Services Tyler.Sadwith@dhcs.ca.gov | Paula Wilhelm Deputy Director Behavioral Health Department of Health Care Services Paula.Wilhelm@dhcs.ca.gov |
| Lindy Harrington Assistant State Medicaid Director Department of Health Care Services Lindy.Harrington@dhcs.ca.gov |
Finding 1 The Department of Health Care Services (DHCS) Licenses and Certifies Treatment Facilities Appropriately, But Its Inspections Have Been Late Since the Pandemic
Recommendation 1
To ensure that it inspects all treatment facilities as required by state law, DHCS should do the following by October 2025:
- Provide management with information about the timeliness of compliance inspections. Such reporting should include the license expiration date for each facility, the status of the inspections, and the date by which the inspections must be completed to meet the 90-day inspection target.
- Implement a mechanism in its licensing database that notifies its staff of the dates for upcoming compliance inspections for their caseload so they can plan accordingly.
- Fill its vacant positions.
DHCS’ Response:
The Department of Health Care Services is actively making changes to current processes to ensure all the following are complete by October 2025:
- In 2023, DHCS received two additional Licensing supervisor positions to assist with the support of the Licensing analysts during the application and compliance review processes. DHCS Licensing supervisors will provide direct management information regarding the timeliness of inspections through the implementation of the following corrective actions: (1) DHCS Licensing supervisors will run weekly reports of active programs and applications that filter data based on facilities and programs in need of inspections within their two-year window. Managers will be able to review those reports and notify staff of site visit needs and deadlines, (2) DHCS’s compliance analysts will include previous compliance visit dates to the individual’s caseload tracking log to monitor due dates for assigned facilities and programs, and (3) DHCS will provide training for all Licensing staff on the new tools and expectations to ensure the new measures are utilized. Enhancement processes will allow Licensing to closely monitor the timeliness of inspections to ensure inspections occur within the required timelines.
- In 2025, DHCS will begin using two new digital platforms; both are currently in development through collaboration between Licensing and Information Technology (IT). The first digital platform, entitled “Survey 123,” will be used during onsite inspections to create digital reports and dashboards for analysts and supervisors to quickly review and use to approve the onsite inspection reports to aid DHCS in sending providers inspection reports faster, improving communication, and accelerating the corrections process between DHCS staff and providers.
The second digital platform will speed up the processing time for licensing and certifying initial applications, renewals, and change requests, thereby decreasing the amount of staff time required to review manual paperwork. This platform will include a database that will store historical provider information, which will improve the fidelity of the information and make it more easily accessible to staff working remotely or in the office. The implementation of this new digital platform will improve monitoring and notification systems to allow DHCS to implement processes for notifying staff of upcoming inspections on their caseloads. The automated tracking of necessary inspections will allow staff to plan travel efficiently based on current data and organizational needs.
Overall, both digital platforms will streamline processes, increase staff’s capacity to conduct additional inspections within the mandated timeframe, improve communication, and support more transparency.
- To fill vacant positions, Licensing supervisors will continue to begin the screening applications within one week of being released from Human Resources (HR). Once all applications are screened, the selected candidate names are submitted to HR to ensure they have met the minimum qualifications. Upon confirmation of qualified candidates, supervisors will prioritize scheduling interviews and finalizing the selected candidates. As new analysts are added, they will receive comprehensive training, prior to being approved by the respective supervisor to conduct independent reviews.
Finding 2 DHCS Does Not Always Promptly or Thoroughly Investigate Complaints
Recommendation 2
To ensure that it assigns complaints to analysts for investigation within 10 days as required by regulations, Health Care Services should update its policies and staff training by April 2025 to clarify the requirement.
DHCS’ Response:
To ensure complaints are assigned to analysts for investigation within ten days as required by regulations, DHCS revised the complaint intake process in August 2024 to support timelier assignments of complaints as detailed in the updated Complaints Section Operations Manual (COM) (See substantiation Rec 2 -Complaint Intake Process as of August 2024 document). Analysts were initially trained on August 7, 2024, and two subsequent biweekly complaint staff meetings were held on August 8, 2024, and August 24, 2024, to address questions and provide additional guidance. The policy update resulted in the timely assignment of all qualifying cases by the intake supervisor with assistance from the complaints supervisor team and section chiefs. The intake supervisor assigns all qualifying complaints within seven business days from the date the complaint is received. Supervisors regularly monitor the updated assignment process through unit meetings and one-on-one meetings between analysts and their direct supervisors. The complaints section chiefs also monitor the progress of the updated assignment process on a weekly basis to ensure this solution is sustained.
Recommendation 3
To improve the timeliness of its investigations and align investigations of counselor complaints, which have interim deadlines for key investigation steps, with other complaint types, DHCS should implement guidelines by October 2025 that specify the length of time analysts should take to complete key steps in the investigation process for different types of investigations.
DHCS’ Response:
To improve the timeliness of investigations, DHCS will implement guidelines by October 2025 to specify the targeted timelines analysts have to complete key steps in the investigation process for different types of investigations. DHCS continues to evaluate complaint data to investigate and close complaints timely.
Since 2022, the length of time, from assignment to closure, has decreased for complaint investigations. Adjustments were made to the Master Complaint Log (MCL), which allowed for the collection of additional data elements related to the length of time a complaint has been open and how long a complaint has been assigned to an analyst. This data is reviewed by both complaints management and analysts. The Complaint Section Operations Manual (COM) was updated in March 2022 to include a policy that states, “All Investigative Reports should be submitted to [the analyst’s] supervisor no later than 60 days after the date of travel for onsite investigations or the date of assignment for in-house investigations.” Previously, this policy was not consistently monitored for compliance, which led, in part, to delayed complaint closure times. DHCS will update the policy to include:
- A timeframe during which the report must be returned from the supervisor to the analyst for final review and edits.
- A timeframe for review by the section chief, if applicable (counselors and death investigations only).
These additional updates will assist with the timely processing and closure of complaints.
Additionally, both the complaint section and unit chiefs increased their monitoring of open complaint data to ensure the timeliness of high, medium, and low-priority cases. Unit chiefs continue to review the COM to ensure that all policies are adhered to, and deadlines are met. Further, the unit chiefs monitor individual staff assignments and meet with staff frequently to prioritize site visits and/or report writing, with low-level reports to be submitted for review within ten business days of assignment for cases that do not require an on-site visit or within ten business days from the date of travel, for cases that require an on-site visit. Continuing evaluation and necessary updates will be added to the COM, and any necessary training for all complaints staff will be completed by October 2025.
Recommendation 4
To ensure that it is conducting thorough investigations of unlicensed treatment facilities, DHCS should conduct site visits beginning in December 2024 in all instances in which there is an allegation that an unlicensed facility is advertising or providing treatment services without a license.
DHCS’ Response:
DHCS currently investigates all unlicensed complaints and while DHCS agrees best practice is to conduct site visits in all instances where there is an allegation that an unlicensed facility is advertising or providing licensable services, a site visit is not always conducted because when DHCS receives unlicensed complaints regarding advertising, the complaint frequently does not include an address of a physical location.
Beginning in December 2024, DHCS will begin conducting site visits for unlicensed complaints to the extent feasible. During the investigative process and research, DHCS will make efforts to determine an address or physical location of the facility being advertised or was reported as providing licensable services and will conduct a site visit when an address is identified and document in the complaint records if DHCS was unable to identify the address and/or physical location of the facility.
In May 2022, DHCS transitioned three vacant positions from the Sacramento office to create a regional office to be more responsive and timelier to complaints in the Southern Region. In 2023, the Complaints section received two additional staff resources to assist with complaints in the Southern California region. These Complaints staff will continue to assist in addressing unlicensed complaint activity in a timelier manner due to their physical location in Southern California.
Recommendation 5
To ensure that unlicensed treatment facilities do not continue to provide services without a license after an investigation substantiates the allegation, by April 2025, DHCS should develop and implement a follow-up procedure, such as by performing another site visit, to confirm the unlicensed facility has ceased providing such services.
DHCS’ Response:
DHCS will develop and implement a follow-up procedure by April 2025 for instances in which allegations of a treatment facility providing licensable services without a license are substantiated. Effective April 2025, once a facility has been cited and has informed DHCS that the unlicensed services have ceased, a follow-up visit will be conducted to verify services are no longer being provided in accordance with the established procedure.
- State regulations define detoxification as a service designed to support and to assist an individual in the alcohol or drug withdrawal process and to explore plans for continued service. ↩︎
- In response to state law, Health Care Services increased the application fee to range from $4,392 to $5,858, which became effective July 1, 2024. ↩︎
- The certification for nonresidential treatment facilities will soon no longer be voluntary. The Legislature passed a law requiring that nonresidential treatment facilities obtain certification by January 1, 2025. Certification remains voluntary for licensed facilities. ↩︎
- State law allows for a separate licensing option so that treatment facilities may spread residents or services across multiple buildings under a single license. However, this type of license does not allow facilities to exceed their approved number of residents or avoid local zoning requirements if they have more than six residents total across those buildings. ↩︎
- During this period, Health Care Services received an average total of 272 initial license and certification applications annually. ↩︎
- Although Health Care Services’ practice is to not announce inspections, it announced inspections ahead of time when it conducted virtual inspections during the pandemic. ↩︎
- This applies for most deficiencies, unless the analyst determines that the deficiency is sufficiently serious to require correction within a shorter period. For the most serious deficiencies, the facility must eliminate or correct the deficiency immediately upon receipt of the notice of deficiency. ↩︎
- We initially selected 30 treatment facilities to review. However, four facilities ceased operations before the end of their licensure period, and as a result, Health Care Services could not conduct inspections on those closed facilities. ↩︎
- During this time, Health Care Services conducted virtual video visits. ↩︎
- Health Care Services is responsible for investigating the deaths of treatment facility residents. The department’s internal death investigation processes were not required in this instance because the individual was not a resident or a program participant. ↩︎